健身增肌的原理是什么?
前 言
现代健身活动文化鼓舞人们崇尚多关节,少碳水化合物,崇尚线条分野的皮肤形态和线条,那意味着安康、强健、力量、诱惑力和病态。
图1:力训研究所女成员对增肌的根本原理,绝多对折健身活动发烧友练了那辈子,增肌增了那辈子,都是莫明其妙的,许多健身活动写手/科学普及者也是敬而远之。
听他们讲增肌的根本原理,一会儿“适应才能增粗”,一会儿“失去平衡后皮夏涅恢复”,一会儿“关节没有法子不能不生长”,让人觉得捉急,即便那不包罗任何数据量,跟没说那样。
要把根本原理讲透,那就比力冗杂了。关键字:卵白量造备、机械冲击力、细胞量信号、DNAmRNA。
一、多半健身活动者对卵白量存在误会多半说健身活动者都认为,他们吃下去的卵白量做为原料,来构成他们的关节,那显然不合错误,即便那混为一谈了“卵白量”和“胺基酸”。他们修建皮肤,用的是胺基酸,并不是卵白量。
卵白量由大量的胺基酸和键构成,是内部构造比力冗杂的核酸化学物量[1]。他们举两个具备目标性的规范。第一个,人体血红素(图2)。
图2-1第三个规范,某些原核有机体内的RNA卵白量。
图2-2图2-3各人能够窥见,卵白量具备极为冗杂的内部构造,他们底子不成能将间接利用内部构造如斯冗杂的核酸化学物量来修建他们的皮肤,他们得先把卵白量吸收、分拆。
在吸收和分拆的过程中,人操纵各类淀粉酶,如脂量[2][3][4][5]、肠卵白量酶[6][7][8][9]等,把食物中的卵白量降解[10][11][12]成为最根本单元——胺基酸[13][14][15]、或多个胺基酸构成的短肽[16][17][18],接着才气用它们用来修建他们的皮肤。
二、增肌,事实Lavardac那儿?许多健身活动者都能答复:增的是骨骼肌。
接着呢?没了。
绝多对折人都晓得“增粗骨骼肌”、增加关节中的卵白量造备,但骨骼肌是若何变长的,卵白量造备事实在骨骼肌的那儿,却想欠亨。
对他们来说,起首要弄清晰骨骼肌的内部构造,骨骼肌中的卵白量扩增到了那儿。在一般人的知觉中,细胞量可能将像个球那样,颜值,中间是细胞量核。
图3:细胞量但骨骼肌(骨骼肌就是骨骼肌)并不是个棒状的构成,而是像笔挺铁管那样。骨骼肌外表是细胞量膜,里面次要有更多更细的“铁管”:埃蒙X,它外面包覆着文艳,细胞量里还有实核细胞、细胞量核等等。
图4:关节内部构造他们的关节之所以可以收缩,次要是即便骨骼肌内的埃蒙X,它的内部含有更多更细的“肌丝”:粗肌丝、细肌丝[5,6]。
关节收缩时,在神经系统释放的生物电的刺激下[19][20][21][22],粗细肌丝之间的“锁”被翻开[23][24][25][26][27],ATP氧化释放能量,带动粗/细肌丝彼此“滑行”,关节缩短,完成收缩[28][29]。
肌动卵白量[30][31][32]构成他们骨骼肌中的细肌丝,肌球卵白量[33][34][35][36]构成粗肌丝。例如人心肌中的肌球卵白量复合物:
图5:人心肌中肌球卵白量复合物如今各人应该大白了,所谓增肌,次要增的是埃蒙X上的粗、细肌丝上的卵白量。
三、埃蒙X内的卵白量是怎么来的?许多人简单的认为,骨骼肌中的卵白量不就是吃下去的卵白量合成成胺基酸构成的。那个说法没错,但几乎就是废话,即便胺基酸是若何构成卵白量,那才是关键。
与绝多对折人想象的差别,现实上,卵白量的品种十分多[37][38][39][40]。多到什么水平呢?原核生物一个细胞量内的卵白量,就多达几万种。
卵白量的内部构造比力冗杂[41][42][43],在空间中呈现立体的几何形态[44][45],具备多层的扭曲和折叠性状[46][47][48][49][50];只要略微有一点变革,它的功用、特征和不变性就可能将发作变革[51][52][53]。
图6:胰岛素降解酶图7:IGF-1受体与胰岛素复合物图8:亚硝酸盐复原酶图9:谷氨酸脱氢酶图10:E3毗连酶泛素卵白量的内部构造比力冗杂,当然也包罗他们的关节。人关节里的卵白量是大量胺基酸构成的生物核酸化学物量[54][55][56][57],人关节的肌球卵白量(及其连系物)在仪器的目光下看上去长如许。
图11:人肌球卵白量复合物图12:人肌球卵白量复合物图13:人肌球卵白量复合物因为人体关节中的卵白量内部构造如斯冗杂,那么很显然,大量的胺基酸绝不成能将凭白无故的、在没有“指引”的前提下,就能根据某种预先设置好的体例来修建如斯冗杂的核酸卵白量。
那就像你有大量的砖石,但是用砖石造造建筑,需要设想图,并并不是把砖石胡乱堆在一路就是建筑了。胺基酸构成卵白量也是那样的事理。毫无疑问,有什么工具在引导它们。
谜底是mRNA。mRNA好像一条链子,上面预留了差别类型的胺基酸的连系区(密码子)。皮肤把大量的胺基酸运输过来,每个胺基酸能够对号入座,“组拆”到那条链子上。
图14:mRNA当然,光是组拆还远远不敷。组拆好了以后,那只是构成了卵白量的雏形罢了,卵白量有四级空间内部构造,从宏不雅上看,是多重折叠的。例如人体血红素,3D看是如许:
 图15:人体血红素
图15:人体血红素但是,人体血红素,若是用图形暗示,在教科书上,是如许的:
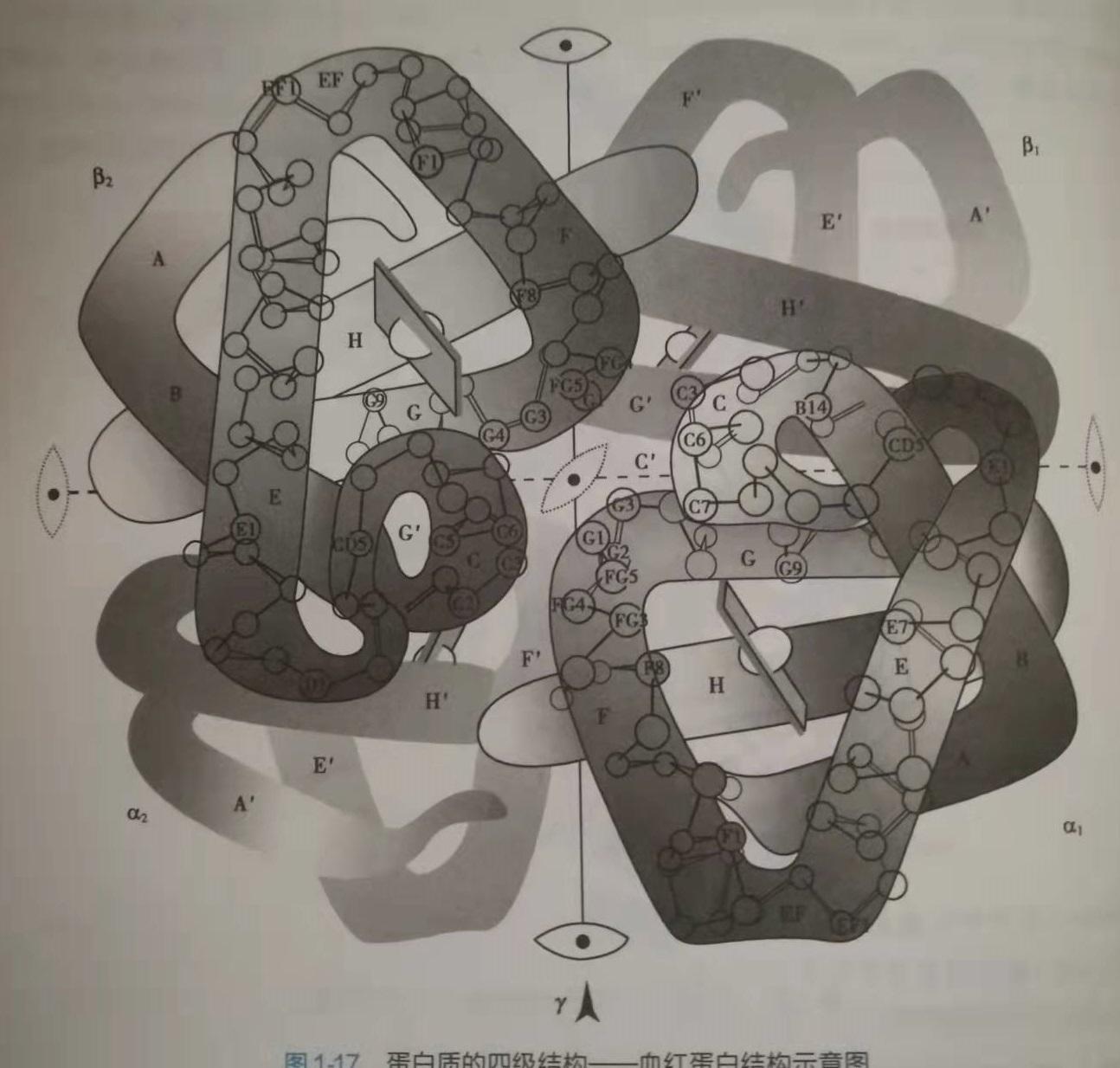 图16:人体血红素
图16:人体血红素仅仅胺基酸“组拆”到mRNA上还不敷,还有折叠[58][59][60][61]、润色[62][63][64][65]、转运[66][67]等许多工做要做;他们把mRNA酿成卵白量(的雏形)那一步,叫做翻译[68][69][70][71][72]。
那mRNA哪来的呢?是DNA以它本身为模板,复造出来的(单螺旋内部构造)。那一部叫做mRNA[73][74][75][76];从DNA到卵白量,宏不雅上次要是mRNA和翻译那两步。
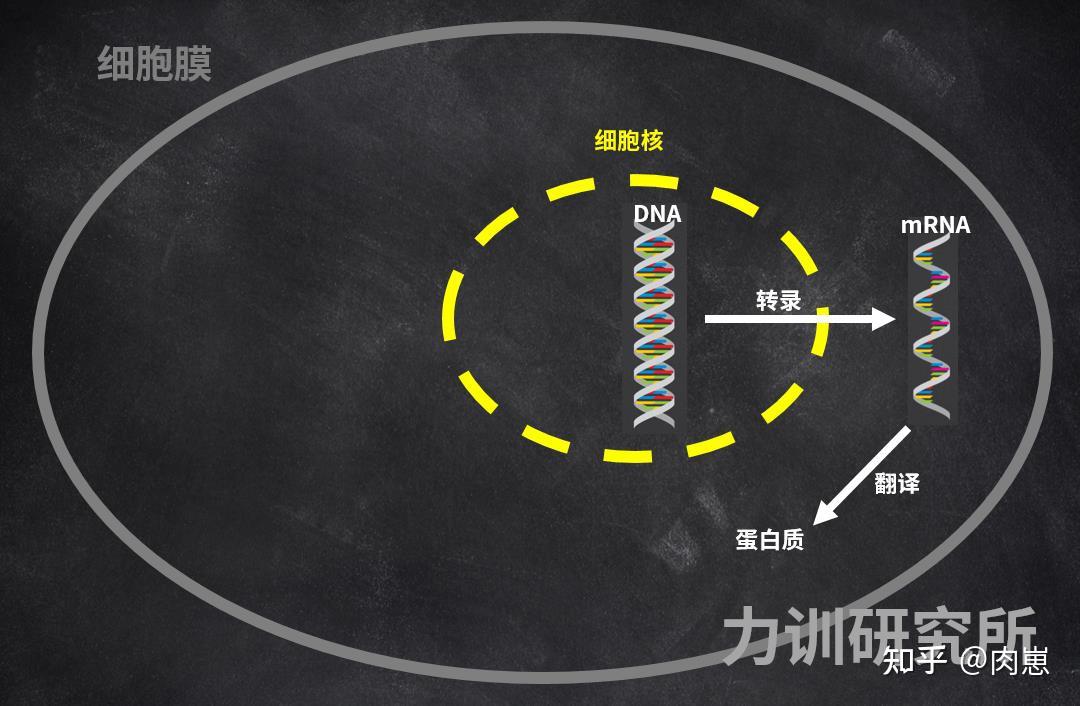 图17:DNA的表达
图17:DNA的表达每种卵白量都有对应的DNA。
若是他们把卵白量视为产物,那么DNA就是设想图,卵白量是根据DNA造出来的[77][78][79][80][81][82][83]。好比他们运输氧气的血红素[84][85]就是生物操纵DNA编码出来的[86],人体内无限多种的卵白量、酶、皮肤内部构造,都是如斯。
他们的每个细胞量不竭凋亡,新的细胞量不竭产生,那个产生过程,都是DNA表达的成果。他们也能够说,新陈代谢是依靠DNA来停止的,DNA是生命活动的中心。
 图18:DNA——活动的中心四、训练:刺激DNA表达
图18:DNA——活动的中心四、训练:刺激DNA表达
他们在前面说了,他们的关节中的卵白量(肌动卵白量/肌球卵白量等)是大量胺基酸以特定体例摆列组合而成的。胺基酸的摆列组合,依靠mRNA;mRNA是DNA复造的产品。
所以,卵白量的“造造”过程,最次要是两部:DNAmRNA为mRNA,mRNA翻译为卵白量。
从DNA到卵白量,那也叫基因的表达。
那为什么DNA会起头mRNA?谜底是,有什么工具刺激了它。好比训练,一种施加在骨骼肌上的机械外力,也叫机械冲击力。张,望文生义,把骨骼肌往两边张开、拉开、扯开。
例如在哑铃弯举中,重力感化于哑铃,哑铃把骨骼肌往下“扯”,他们本身的骨骼支持,把骨骼肌往上拉,则构成了一个往两边张开的力。
 图19:机械冲击力
图19:机械冲击力机械冲击力为什么能刺激DNA表达[87][88][89](mRNA)呢?即便他们有大量的生物感触感染器,能把外力信号,改变为细胞量内的生物信号,那些信号不断传递到DNA上,刺激了DNA的mRNA,于是他们得到了骨骼肌内的卵白量。
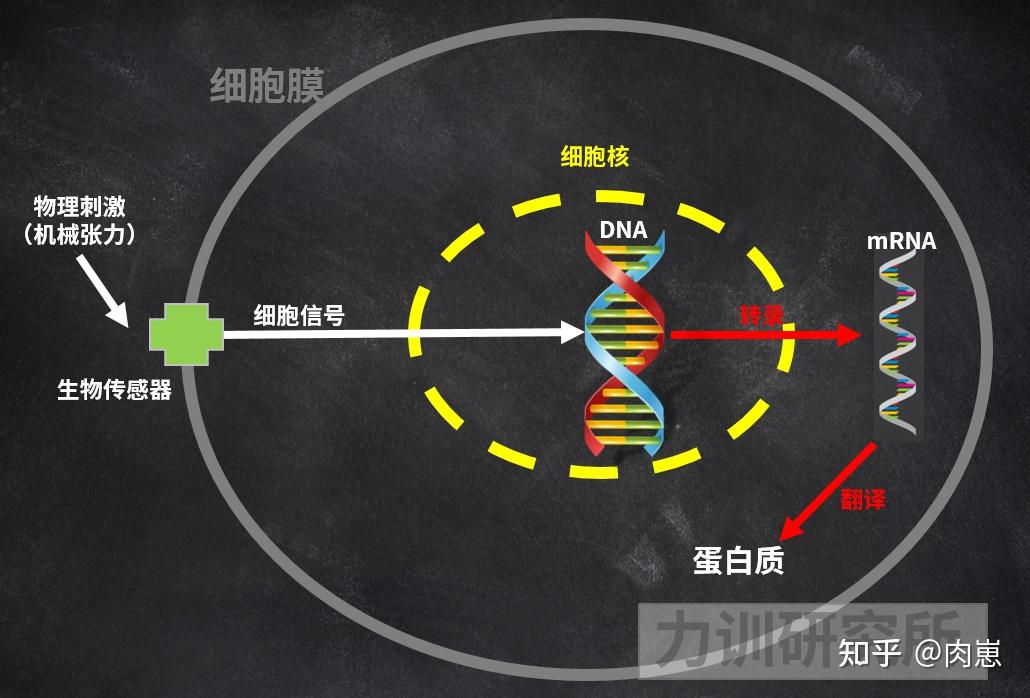 图20:机械刺激与细胞量信号
图20:机械刺激与细胞量信号关节上能感知机械冲击力的生物传感器有哪些呢?
例如肋节[90][91][92],它将骨骼肌膜与埃蒙X、细胞量外基量毗连起来,加强骨骼肌膜的不变性和强度,还能感触感染、侦测到施加于及细胞量的外力(例如他们所说的机械冲击力),将其传导到骨骼肌内部,转化为生物信号[16,17];
71整合素[93][94]也是一种横跨细胞量膜的受体,它一方面供给毗连感化[95],一方面将机械信号从细胞量别传递到细胞量内。还有磷脂酸(PA)、FAK—粘着斑激酶等也参与机械冲击力转化为细胞量信号的传导传导,就不多说了。
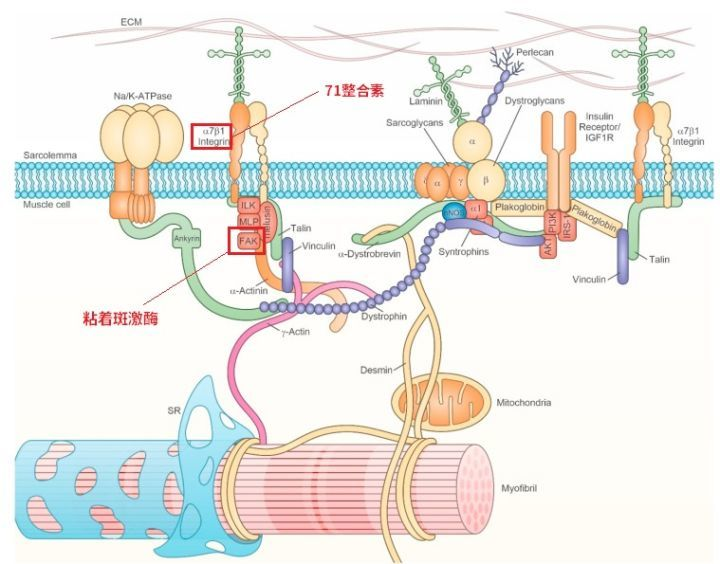 图21:生物传感器五、训练是若何刺激DNA表达的?谜底是细胞量信号
图21:生物传感器五、训练是若何刺激DNA表达的?谜底是细胞量信号
生物感触感染器将细胞量信号传递到细胞量内,引发一系列细胞量信号事务。此中最出名、最核心的细胞量信号事务,也被称为PI3K/Akt/mTOR途径[96][97][98][99][100]。还有一些此外相对次要的途径(如ERK),碍于篇幅,他们就不在那里说了。
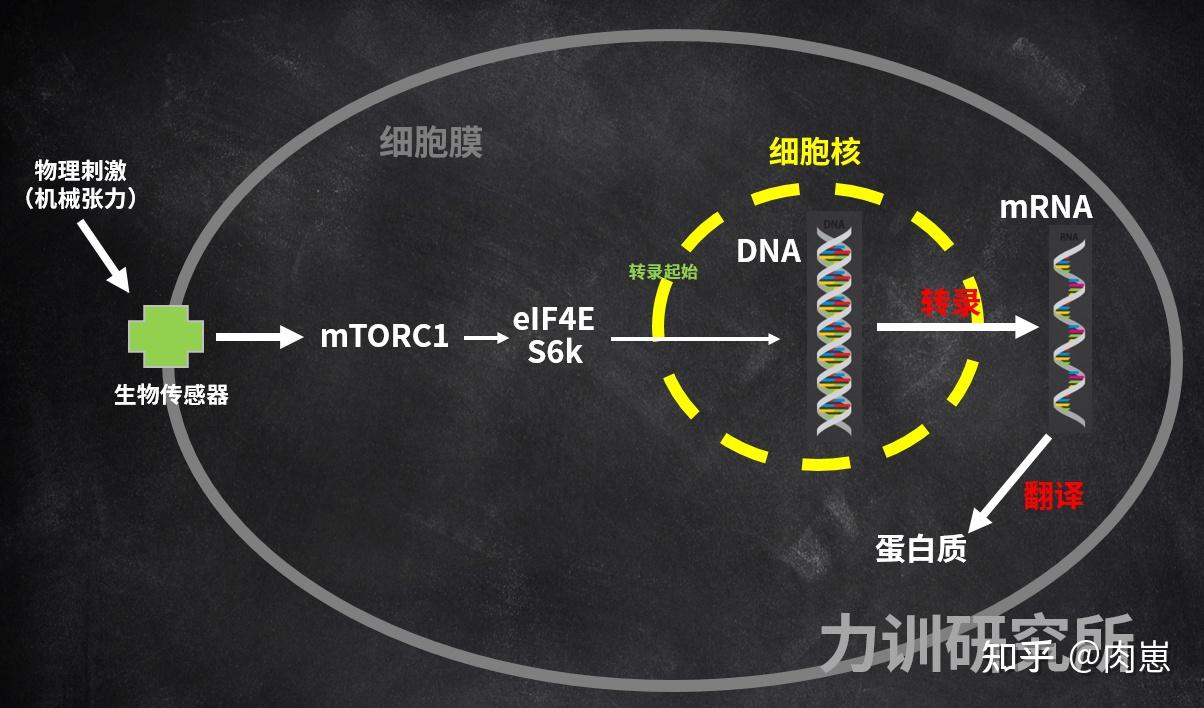 图22:增肌的核心—mTOR途径
图22:增肌的核心—mTOR途径mTOR是他们细胞量内一种由2549个胺基酸构成的大卵白量[101],——它既是卵白量,也阐扬信号感化。
mTOR全称“哺乳动物雷帕霉素靶卵白量”,是哺乳动物调理细胞量生长、代谢、卵白量造备等关键心理过程中的重要卵白量[102]。mTOR现实上以mTORC1(复合物1)和mTORC2(复合物2)的形式在人体内存在[103]。
 图23:mTORC1
图23:mTORC1MTORC1次要通过S6K1和eIF4E的磷酸化,来引发DNA表达,促进卵白量造备[104]。此外,S6k1也进步mRNA的翻译效率[105]。
对健身活动者来说,最典型的激活mTORC1的因素,当然是训练。
训练刺激(机械冲击力)可激活mTORC1,使其磷酸化[106][107][108][109][110][111];在mTOR的下流,S6激酶[112][113][114]和eIF4E(原核生物起始因子4E)[115][116]随之也被磷酸化(红色方框)。
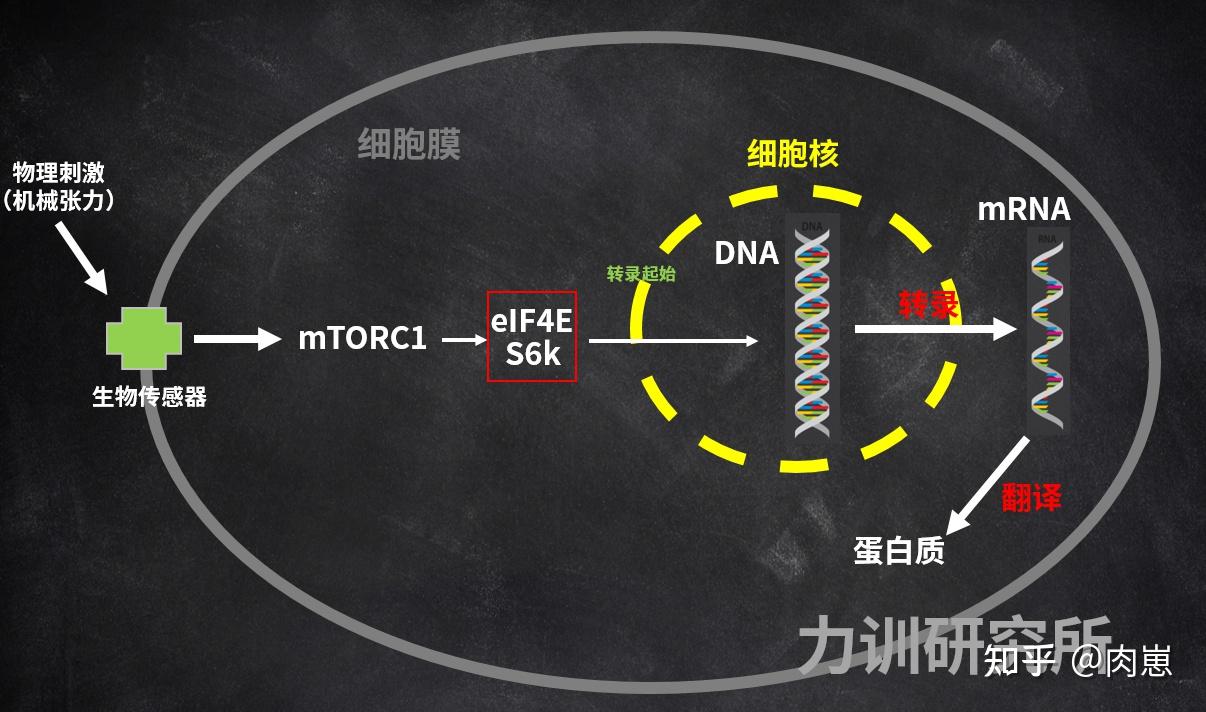 图24:机械冲击力传递到DNA,引发DNA表达
图24:机械冲击力传递到DNA,引发DNA表达磷酸化是天然界一种十分遍及的、对卵白量停止化学润色的过程[117]。卵白量磷酸化有效地增加了其冗杂性,远远超越了基因组所付与的多样性[118]。
在磷酸激酶的感化下,生物将磷酸基团加在卵白量或卵白量类中间产品上,从而将卵白量磷酸化(或者去磷酸化)。经化学润色后的卵白量,功用/生物活性会显著差别。
目前已知的磷酸激酶多大500多种,可针对超越20000种卵白量上的25000个点位停止磷酸化[119][120][121][122];磷酸化决定了在一般/病理形态下有机体对刺激的反响[123]。
S6k1是DNAmRNA因子[124],名至实归。S6k1被激活后,接下来RNA卵白量6被磷酸化,从而增加了RNA卵白量与5‘端寡核苷酸(5’-top)mRNA的亲和力,引起了DNAmRNA[125][126][127],增加卵白量造备[128][129][130][131]。
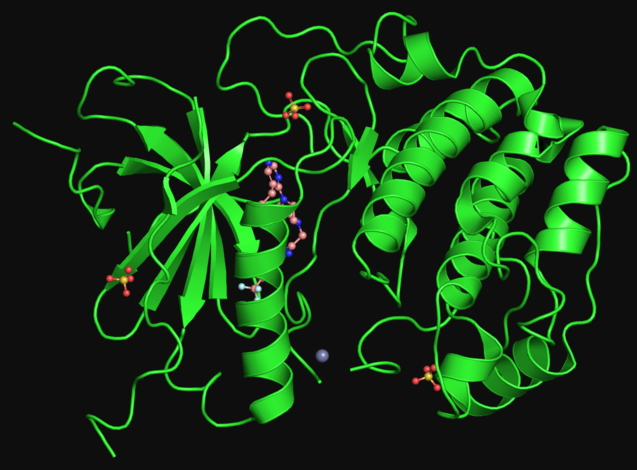 图25:S6k1
图25:S6k1反过来,若是卵白量摄入不敷,胺基酸/原料不敷,则能够招致RNA卵白量6的“去磷酸化”[132]。已知的诱导卵白量去磷酸化的酶超越150种[119]。
研究发现,S6k它的磷酸化程度与增肌之间,存在极强的正相关性,r=0.998。下图纵轴是关节增加的幅度,横轴是s6k磷酸化程度,用俗话来说,它们几乎成反比。
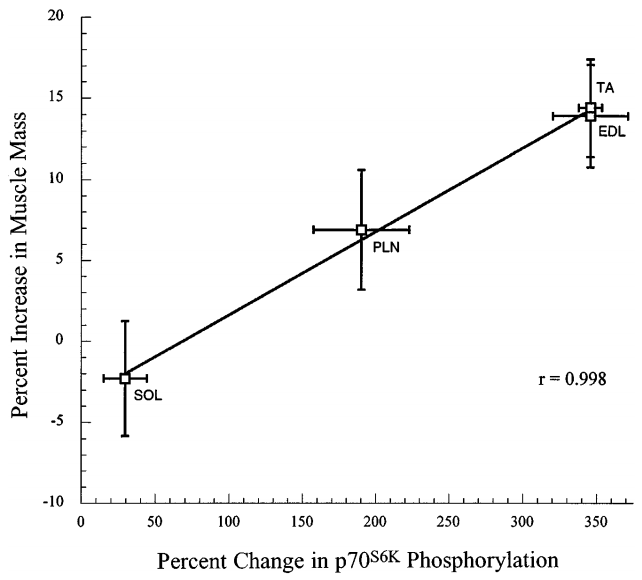 图26:S6k磷酸化与增肌六、能激活mTOR途径的,不行有训练
图26:S6k磷酸化与增肌六、能激活mTOR途径的,不行有训练
S道上有一种典型的错误概念,认为训练才气增肌,好比下面图上的那种:
 图27:错误概念
图27:错误概念留意,图中有2个错误:
错误1:认为增肌的根本原理是损伤修复。那个错误他们在前面已经解析过了,增肌的次要根本原理是DNA表达而并不是损伤。民间认为损伤增肌,次要是即便损伤是来自于训练,训练能激发DNA表达。
错误2:认为训练是增肌的前提。那也是错的,除了训练,营养[133][134][135][136][137][138][139][140][141]和激素也都能激发DNA表达,即便他们的分子途径是高度类似的,根本原理也不异:都是是通过刺激DNA的表达,来得到更多的肌卵白量。
当然,它们的效果水平差别,那或许是即便三者招致的mTOR磷酸化水平不那样。
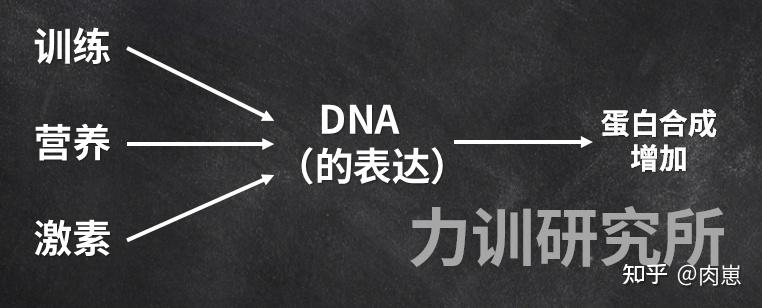 图28
图28他们关节中的卵白量就是那么来的:从激活细胞量膜上的受体起头,一个个卵白量/酶依次被激活,最初激活DNAmRNA,接着mRNA翻译为卵白量。
许多人很难承受“只打药不练就能够长关节”,若是他们晓得“只吃卵白量粉不训练也能长关节”,估量就更无法承受了。但那是客不雅事实,不以他们的主不雅意志为转移。
Liu等人在《临床内排泄与代谢杂志》上颁发的了一项以10名安康年轻报酬对象的研究,对他们**胺基酸,察看他们骨骼肌内的变革(图30),灰柱“AA”是**胺基酸后,白色是**前。纵轴是磷酸化程度。
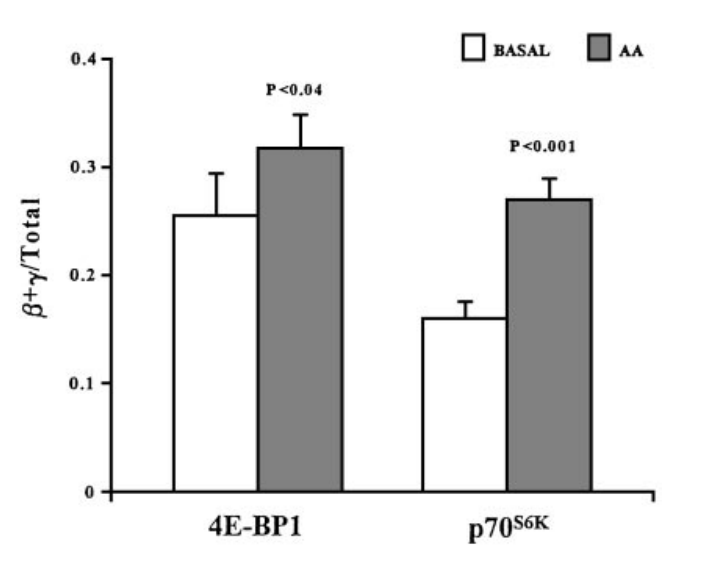 图30
图30胺基酸**,招致p70S6K和eIF4E磷酸化,进而增加了RNA卵白量S6的磷酸化。S6被磷酸化后,促进了一些在卵白量造备中起重要感化的RNA卵白量的造备。
那些,也就证了然胺基酸**或摄入能磷酸化4E-BP1、eIF4E、S6,进而引发卵白量造备:换句话说,单纯的吃卵白量不运动,也会多几少长一些关节。
除了饮食/营养/训练三者,他们还列举了第四种刺激影响mRNA因子磷酸化的要素:光。
证据表白,光通过刺激SCN(视穿插上核)来影响ERK、或mTOR途径的下流因子S6核糖激酶的磷酸化。详细的,在他们之前的文章中有过论述。
保举阅读肉崽:有哪些是你健身活动久了晓得的事?
参考^Frauenfelder H; Sligar SG; Wolynes PG The energy landscapes and motions of proteins. Science 1991, 254, 1598–1603.^Farhath S, He Z, Nakhla T, et al. Pepsin, a marker of gastric contents, is increased in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatrics 2008;121:253–259.^Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med 2007;175:1298–1303.^Crapko M, Kerschner JE, Syring M, Johnston N. Role of extra‐esophageal reflux in chronic otitis media with effusion. Laryngoscope 2007;117:1419–1423.^Knight J, Lively MO, Johnston N, Dettmar PW, Koufman JA. Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope 2005;115:1473–1478.^Burkhart J.M., Schumbrutzki C., Wortelkamp S., Sickman A., Zahedi R.P. Systematic and quantitative comparison of digest efficiency and specificity reveals the impact of trypsin quality on MS-based proteomics. J. Proteom. 2012;75:1454–1462.^Olsen J.V., Ong S.E., Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteom. 2004;3:608–614.^Keil B. Trypsin. Enzymes. 1971;3:249–275.^Rodriguez J., Gupta N., **ith R.D., Pevzner P.A. Does trypsin cut before proline? J. Proteom. Res. 2008;7:300–305.^Matthews DM. Protein absorption. J Clin Pathol Suppl (R Coll Pathol) 1971;5:29–40.^Matthews DM. Intestinal absorption of peptides. Physiol Rev. 1975 Oct;55(4):537–608.^Mathews DM, Adibi SA. Peptide absorption. Gastroenterology. 1976 Jul;71(1):151–161.^Adibi SA. Intestinal transport of dipeptides in man: relative importance of hydrolysis and intact absorption. J Clin Invest. 1971 Nov;50(11):2266–2275.^Adibi SA. The influence of molecular structure of neutral amino acids on their absorption kinetics in the jejunum and ileum of human intestine in vivo. Gastroenterology. 1969 May;56(5):903–913.^Adibi SA, Gray SJ. Intestinal absorption of essential amino acids in man. Gastroenterology. 1967 May;52(5):837–845.^Silk DB. Progress report. Peptide absorption in man. Gut. 1974 Jun;15(6):494–501.^Adibi SA, Morse EL. The number of glycine residues which limits intact absorption of glycine oligopeptides in human jejunum. J Clin Invest. 1977 Nov;60(5):1008–1016.^Kania RK, Santiago NA, Gray GM. Intestinal surface amino-oligopeptidases. II. Substrate kinetics and topography of the active site. J Biol Chem. 1977 Jul 25;252(14):4929–4934.^Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261:58–65.^Rayment I, Rypniewski WR, Schmidt-B?se K, **ith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM (1993) Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261:50–58.^R Dabrowska, W Drabikowski.Molecular basis of muscular contraction.Postepy Biochem. 1973;19(3):343-59.^Postepy Biochem.The cross-bridge theory. Journal: Physiological 1973;19(3):343-59.^Thorson J, White DC. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390.^Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994 Dec;67(6):2422–2435.^R D Bremel, A Weber.Cooperation within actin filament in vertebrate skeletal muscle.Nat New Biol. 1972 Jul 26;238(82):97-101.^Ricarda Haeger , Felipe de Souza Leite , Dilson E Rassier.Sarcomere length non-uniformities dictate force production along the descending limb of the force-length relation.Proc Biol Sci. 2020 Oct 28;287(1937):20202133.^Dilson E Rassier.Sarcomere mechanics in striated muscles: from molecules to sarcomeres to http://cells.Am J Physiol Cell Physiol. 2017 Aug 1;313(2):C134-C145.Epub 2017 May 24.^Kiisa Nishikawa 1 , Samrat Dutta 2 , Michael DuVall 2 3 , Brent Nelson 4 , Matthew J Gage 5 , Jenna A Monroy 6.Calcium-dependent titin-thin filament interactions in muscle: observations and theory.J Muscle Res Cell Motil. 2020 Mar;41(1):125-139.Epub 2019 Jul 9.^ B Brenner, E Eisenberg.The mechanism of muscle contraction. Biochemical, mechanical, and structural approaches to elucidate cross-bridge action in muscle.Basic Res Cardiol. 1987;82 Suppl 2:3-16.^ Maruyama K, Ebashi S. α-Actinin, a new structural protein from striated muscle. II. Action on actin. J Biochem. 1965;58:13–19.^Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140:1383–1393.^Carlier MF, Valentin-Ranc C, Combeau C, Fievez S, Pantoloni D. Actin polymerization: regulation by divalent metal ion and nucleotide binding, ATP hydrolysis and binding of myosin. Adv Exp Med Biol 358: 71–81, 1994^Almenar-Queralt A, Lee A, Conley CA, Ribas de Pouplana L, Fowler VM. Identification of a novel tropomodulin isoform, skeletal tropomodulin, that caps actin filament pointed ends in fast skeletal muscle. J Biol Chem 274: 28466–28475, 1999^Broschat KO, Weber A, Burgess DR. Tropomyosin stabilizes the pointed end of actin filaments by slowing depolymerization. Biochemistry 28: 8501–8506, 1989^Fowler VM, Sussmann MA, Miller PG, Flucher BE, Daniels MP. Tropomodulin is associated with the free (pointed) ends of the thin filaments in rat skeletal muscle. J Cell Biol 120: 411–420, 1993^Gokhin DS, Lewis RA, McKeown CR, Nowak RB, Kim NE, Littlefield RS, Lieber RL, Fowler VM. Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology. J Cell Biol 189: 95–109, 2010^Lau KF, Dill KA. Theory for protein mutability and biogenesis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):638–642^Tsai CJ; Ma B; Sham YY; Kumar S; Nussinov R Structured disorder and conformational selection. Proteins 2001, 44, 418–427.^Tzeng SR; Kalodimos CG Protein dynamics and allostery: an NMR view. Curr. Opin. Struct. Biol 2011, 21, 62–67.^Zhuravlev PI; Papoian GA Protein functional landscapes, dynamics, allostery: a tortuous path towards a universal theoretical framework. Q. Rev. Biophys 2010, 43, 295–332.^Chan HS, Dill KA. Origins of structure in globular proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6388–6392.^Woodward CK, Rosenberg A. Studies of hydrogen exchange in proteins. VI. Urea effects on ribonuclease exchange kinetics leading to a general model for hydrogen exchange from folded proteins. J Biol Chem. 1971 Jul 10;246(13):4114–4121.^Shortle D, Chan HS, Dill KA. Modeling the effects of mutations on the denatured states of proteins. Protein Sci. 1992 Feb;1(2):201–215^Venyaminov SY; Rajnavolgyi E; Medgyesi GA; Gergely J; Zavodszky P The role of interchain disulphide bridges in the conformational stability of human immunoglobulin G1 subclass. Hydrogen-deuterium exchange studies. Eur. J. Biochem 1976, 67, 81–86.^Qin H; Lim L; Song J Protein dynamics at Eph receptor-ligand interfaces as revealed by crystallography, NMR and MD simulations. BMC Biophys. 2012,5,2.^Karplus M; Weaver DL Protein-folding dynamics. Nature 1976, 260, 404–406.^McCammon JA; Gelin BR; Karplus M Dynamics of folded proteins. Nature 1977, 267, 585–590.^Karplus M The Levinthal paradox: yesterday and today. Fold. Des 1997, 2, S69–75.^Dill KA. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155.^Dill KA, Bromberg S, Yue K, Fiebig KM, Yee DP, Thomas PD, Chan HS. Principles of protein folding--a perspective from simple exact models. Protein Sci. 1995 Apr;4(4):561–602.^Ackers GK, Doyle ML, Myers D, Daugherty MA. Molecular code for cooperativity in hemoglobin. Science. 1992 Jan 3;255(5040):54–63.^Alonso DO, Dill KA. Solvent denaturation and stabilization of globular proteins. Biochemistry. 1991 Jun 18;30(24):5974–5985.^Shi L, Palleros DR, Fink AL. Protein conformational changes induced by 1,1-bis(4-anilino-5-naphthalenesulfonic acid): preferential binding to the molten globule of DnaK. Biochemistry. 1994 Jun 21;33(24):7536–7546.^Almenar-Queralt A, Lee A, Conley CA, Ribas de Pouplana L, Fowler VM. Identification of a novel tropomodulin isoform, skeletal tropo-modulin, that caps actin filament pointed ends in fast skeletal muscle. J Biol Chem. 1999;274:28466–28475.^Cox PR, Zoghbi HY. Sequencing, expression analysis, and mapping of three unique human tropomodulin genes and their mouse orthologs. Genomics. 2000;63:97–107.^Fowler VM. Identification and purification of a novel Mr 43,000 tropomyosin-binding protein from human erythrocyte membranes. J Biol Chem. 1987;262:12792–12800.^ Weber A, Pennise CR, Babcock GG, Fowler VM. Tropomodulin caps the pointed ends of actin filaments. J Cell Biol. 1994;127:1627–1635.^Anfinsen C.B., Haber E., Sela M., White F.H. The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc. Natl. Acad. Sci. USA. 1961;47:1309–1314.^Anfinsen C.B. Principles that govern the folding of protein chains. Science. 1973;181:223–230.^Svetlov M.S., Kommer A., Kolb V.A., Spirin A.S. Effective cotranslational folding of firefly luciferase without chaperones of the Hsp70 family. Protein Sci. 2006;15:242–247.^Kolb V.A., Makeyev E.V., Spirin A.S. Folding of firefly luciferase during translation in a cell-free system. EMBO J. 1994;13:3631–3637.^Neurath, H. 1989. Proteolytic processing and physiological regulation. Trends Biochem. Sci. 14:268‐271.^Hirschberg, C.B. and Snider, M.D. 1987. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 56:63‐89.^ Suttie, J.W. 1985. Vitamin K–dependent carboxylase. Annu. Rev. Biochem. 54:459‐477.^Kaufman, R.J. , Murtha, P. , Ingolia, D.E. , Yeung, C.Y. , and Kellems, E.R. 1986. b. Selection and amplification of heterologous genes encoding adenosine deaminase in mammalian cells. Proc. Natl.^Rothman, M.E. 1994. Mechanisms of intracellular protein transport. Nature 372:55‐63.^Rothman, J.E. and Orci, L. 1992. Molecular dissection of the secretory pathway. Nature 335:409‐415.^KERR IM, MARTIN EM, HAMILTON MG, WORK TS. The initiation of virus protein synthesis in Krebs ascites tumor cells infected with EMC virus. Cold Spring Harb Symp Quant Biol. 1962;27:259–269.^ Mathews M, Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338.^**ith AE, Marcker KA, Mathews MB. Translation of RNA from encephalomyocarditis virus in a mammalian cell-free system. Nature. 1970 Jan 10;225(5228):184–187.^Leader DP, Klein-Bremhaar H, Wool IG. Distribution of initiation factors in cell fractions from mammalian tissues. Biochem Biophys Res Commun. 1972 Jan 14;46(1):215–224.^Aviv H, Boime I, Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307.^GIACOMONI D, SPIEGELMAN S. Origin and biologic individuality of the genetic dictionary. Science. 1962 Dec 21;138(3547):1328–1331.^BELOZERSKY AN, SPIRIN AS. A correlation between the compositions of deoxyribonucleic and ribonucleic acids. Nature. 1958 Jul 12;182(4628):111–112.^Marmur J, Lane D. STRAND SEPARATION AND SPECIFIC RECOMBINATION IN DEOXYRIBONUCLEIC ACIDS: BIOLOGICAL STUDIES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):453–461.^Ycas M, Vincent WS. A RIBONUCLEIC ACID FRACTION FROM YEAST RELATED IN COMPOSITION TO DESOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1960 Jun;46(6):804–811.^Aviv H, Boime I, Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307.^Boime I, Aviv H, Leder P. Protein synthesis directed by encephalomyocarditis virus RNA. II. The in vitro synthesis of high molecular weight proteins and elements of the viral capsid. Biochem Biophys Res Commun. 1971 Nov 5;45(3):788–795.^Heywood SM, Thompson WC. Studies on the formation of the initiation complex in eukaryotes. Biochem Biophys Res Commun. 1971 May 7;43(3):470–475.^Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448.^McCarthy BJ, Holland JJ. Cultured mammalian cell deoxyribonucleic acid as a template for in vitro protein synthesis. Biochemistry. 1966 May;5(5):1633–1637.^Nathans D. Cell-free protein synthesis directed by coliphage MS2 RNA: synthesis of intact viral coat protein and other products. J Mol Biol. 1965 Sep;13(2):521–531.^McDowell MJ, Joklik WK. An in vitro protein synthesizing system from mouse L fibroblasts infected with reovirus. Virology. 1971 Sep;45(3):724–733.^H A ITANO.Human hemoglobin.Science.1953 Jan 30;117(3031):89-94.^Benz EJ Jr, Forget BG. Defect in messenger RNA for human hemoglobin synthesis in beta thalassemia.The Journal of Clinical Investigation, 01 Dec 1971, 50(12):2755-2760^Stephen Welle 1 , Kirti Bhatt, Carl A Pinkert.Myofibrillar protein synthesis in myostatin-deficient http://mice.Am J Physiol Endocrinol Metab. 2006 Mar;290(3):E409-15.^Neil Kubica 1 , Douglas R Bolster, Peter A Farrell, Scot R Kimball, Leonard S Jefferson.Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner.2005 Mar 4;280(9):7570-80.^Dreyer HC, Fugita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen B (2006) Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576:613–624^Baar K, Esser K (1999) Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276:C120–C127^Luisa Gorza; Matteo Sorge; Laura Seclì; Mara Brancaccio.Master Regulators of Muscle Atrophy: Role of Costamere Components.Cells 2021, 10(1), 61.^Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990;345:315–9.^Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol 2002;18:637–706.^Di Mauro D, Gaeta R, Arco A, Miliardi D, Lentini S, Runci M, et al. Distribution of costameric proteins in normal human ventricular and atrial cardiac muscle. Folia Histochem Cytobiol 2009;47(4):605–8.^Vatta M, Sinagra G, Brunelli L, Faulkner G. Remodeling of dystrophin and sarcomeric Z-band occurs in pediatric cardiomyopathies: a unifying mechanism for force transmission defect. J Cardiovasc Med (Hagerstown) 2009;10(2):149–56.^Berthier C, Blaineau S. Supramolecular organization of the subsarcolemmal cytoskeleton of adult skeletal muscle fibers. A review. Biol Cell 89: 413–434, 1997.^Dreyer HC, Fugita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen B (2006) Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576:613–624^Kubica N, Bolster DR, Farrell PA, Kimball SR, JeVerson LS (2005) Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2B mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280:7570– 7580.^Atherton PJ, Babraj J, **ith K, Singh J, Rennie MJ, Wackerhage H (2005) Selective activation of AMPK-PGC-1alpha or PKBTSC2-mTOR signaling can explain speciWc adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19:786–788^Nader GA (2005) Molecular determinants of skeletal muscle mass: getting the AKT together. Int J Biochem Cell Biol 37:1985–1996^Tidball JG (2005) Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol 98:1900–1908^Sabers CJ, Martin MM, Brunn GJ et al.Isolation of a Protein Target of the FKBP12-Rapamycin Complex in Mammalian Cells.The Journal of biological chemistry. 1995;270(2):815–22.^Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976.^Kim D-H, Sarbassov DD, Ali SM et al.mTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex that Signals to the Cell Growth Machinery.Cell. 2002;110(2):163–75.^ Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580.^Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–313.^Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014 – 1019, 2001.^Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y, Chen J, Hornberger TA. A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell 21: 3258 –3268, 2010.^Hornberger TA, McLoughlin TJ, Leszczynski JK, Armstrong DD, Jameson RR, Bowen PE, Hwang ES, Hou H, Moustafa ME, Carlson BA, Hatfield DL, Diamond AM, Esser KA. Selenoprotein-deficient transgenic mice exhibit enhanced exercise-induced muscle growth. J Nutr 133: 3091–3097, 2003.^Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signaling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 380: 795–804, 2004.^Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280: 7570 –7580, 2005.^Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol 589.7: 1831–1846, 2011.^Pallafacchina, G., Calabria, E., Serrano, A. L., Kalhovde, J. M.,& Schiaffino, S. (2002). A protein kinase B-dependent and rapamycin- sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. U.S.A.,25.^Inoki, K., Li, Y., Zhu, T., Wu, J., & Guan, K. L. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling.Nat. Cell. Biol., 4, 648–657.^Hannan, K. M., Thomas, G., & Pearson, R. B. (2003). Activation of S6K1 (p70 ribosomal protein S6 kinase 1) requires an initial calcium-dependent priming event involving formation of a highmolecular-mass signalling complex. Biochem. J., 370, 469–477.^Kimball SR, Jefferson LS, Fadden P, Haystead TAJ, Lawrence JC 1996 Insulin and diabetes cause reciprocal changes in the association of eIF-4E and PHAS-I in rat skeletal muscle. Am J Physiol 270:C705–C709.^Pause A, Belsham G, Gingras AC, Donze O, Lin TA, Lawrence JC, Sonenberg N 1994 Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5-cap function. Nature 371:762–767.^C S Rubin, O M Rosen.Protein phosphorylation.Annu Rev Biochem. 1975;44:831-87.^Walsh CT, Garneau-Tsodikova S, and Gatto GJ Jr. (2005). Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem. Int. Ed. Engl 44, 7342–7372.^ab Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, and Mustelin T (2004). Protein tyrosine phosphatases in the human genome. Cell 117, 699–711.^Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, and Sullivan M (2012). PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res 40, D261–270.^ Khoury GA, Baliban RC, and Floudas CA (2011). Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci. Rep 1.^Manning G, Whyte DB, Martinez R, Hunter T, and Sudarsanam S (2002). The protein kinase complement of the human genome. Science 298, 1912–1934.^Pawson T, and Scott JD (2005). Protein phosphorylation in signaling--50 years and counting. Trends Biochem. Sci 30, 286–290.^Satoshi Fujita, Hans C Dreyer, Micah J Drummond, Erin L Glynn, Jerson G Cadenas, Fumiaki Yoshizawa, Elena Volpi, Blake B Rasmussen.Nutrient signalling in the regulation of human muscle protein synthesis.J Physiol. 2007 Jul 15;582(Pt 2):813-23.^Jefferies H B J, Reinhard C, Kozma S C, Thomas G. Rapamycin selectively represses translation of the polypyrimidine tract mRNA family. Proc Natl Acad Sci USA. 1994;91:4441–4445.^Jefferies H B J, Thomas G, Thomas G. Elongation factor-1α mRNA is selectively translated following mitogenic stimulation. J Biol Chem. 1994;269:4367–4372.^Thomas G, Thomas G. Translational control of mRNA expression during the early mitogenic response in Swiss mouse 3T3 cells: identification of specific proteins. J Cell Biol. 1986;103:2137–2144.^Gruner S., Peter D., Weber R., Wohlbold L., Chung M.Y., Weichenrieder O., Valkov E., Igreja C., Izaurralde E. The structures of eIF4E-eIF4G complexes reveal an extended interface to regulate translation initiation. Mol. Cell. 2016;64:467–479.^Kubica, N., Bolster, D. R., Farrell, P. A., Kimball, S. R., & Jefferson,L. S. (2005). Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2B{epsilon} mRNA in a mammalian target of rapamycin-dependent manner.J. Biol. Chem., 280, 7570–7580.^Proud CG, Denton RM 1997 Molecular mechanisms for the control of translation by insulin. Biochem J 328:329–341^Kleijn M, Scheper GC, Voorma HO, Thomas AA 1998 Regulation of translation initiation factors by signal transduction. Eur J Biochem 253:531–544.^Thomas G, Thomas G. Translational control of mRNA expression during the early mitogenic response in Swiss mouse 3T3 cells: identification of specific proteins. J Cell Biol. 1986;103:2137–2144.^S R Kimball.Regulation of translation initiation by amino acids in eukaryotic cells.Prog Mol Subcell Biol . 2001;26:155-84. doi: 10.1007/978-3-642-56688-2_6.^Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E (2004) Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab 287:E1–E7^Douglas Paddon-Jones 1 , Melinda Sheffield-Moore, Xiao-Jun Zhang, Elena Volpi, Steven E Wolf, Asle Aarsland, Arny A Ferrando, Robert R Wolfe.Amino acid ingestion improves muscle protein synthesis in the young and elderly.Am J Physiol Endocrinol Metab. 2004 Mar;286(3):E321-8.^K **ith, J M Barua, P W Watt, C M Scrimgeour, M J Rennie.Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine.Am J Physiol. 1992 Mar;262(3 Pt 1):E372-6.^Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8.^P J Garlick, I Grant.Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids.Biochem J. 1988 Sep 1;254(2):579-84.^Koopman R, Crombach N, Gijsen AP, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009; 90:106Y15.^Jeffery Escobar, Jason W Frank, Agus Suryawan, Hanh V Nguyen, Scot R Kimball, Leonard S Jefferson, Teresa A Davis.Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs.Am J Physiol Endocrinol Metab. 2006 Apr;290(4):E612-21.^J C Anthony, T G Anthony, S R Kimball, T C Vary, L S Jefferson.Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation.J Nutr. 2000 Feb;130(2):139-45.版权声明
本文仅代表作者观点,不代表木答案立场。

