当代年轻人为什么尿酸会高?
注:那篇文章只是介绍尿酸的心理/病理常识;
关于尿酸与健身、尿酸与健身饮食调整的常识,还有很大的段落和篇幅,我还没写出来。
若是本文点赞/喜好足够高,我就会间接在本文后面更新上述内容,有兴趣旁观的伴侣请记得三连,谢谢
小结1、尿酸次要是嘌呤转化来的,所以存眷尿酸一般要存眷嘌呤;
2、尿酸的来源包罗嘌呤的饮食摄入/本身合成增加,去路包罗尿液/肠道排泄;
3、尿酸程度取决于合成 和排泄之间的差值;
4、现有的病理研究认为,尿酸高次要是排泄不顺畅为主;
5、排泄不顺畅的次要原因,是遗传。
尿酸是怎么来的?嘌呤是人体遗传物量核酸的构成部门,也是能量代谢的产品。
各类嘌呤在响应的酶(如黄嘌呤氧化酶、鸟嘌呤脱氨酶、黄嘌呤氧化酶)的感化下被转化为尿酸,所以我们在考虑尿酸程度的时候,次要考虑嘌呤。
嘌呤有两种来源。
一类是吃下去的。因为嘌呤是细胞核酸的构成部门,动动物都含有细胞构造,大大都都有细胞核(留意不是全数,如红细胞就没有细胞核)。若是某些食物所含的嘌呤出格高,就会增加我们体内嘌呤程度。
另一类是本身代谢产生,源于人体内卵白量合成代谢、细胞灭亡、一些化合物合成代谢(如ATP)等。
常见的数据表白人体20%的嘌呤来自于食物,80%来自于内源性生成。
尿酸是怎么排泄的?尿酸次要在肝脏产生(小肠和其他组织也能产生一些),通过肾脏/泌尿系统排泄(排出约2/3)[59,60],次要通过肠道排泄(排出约1/3)[24]。
尿酸一边产生,一边排泄,血液尿酸程度取决于两者之差。
若是产生=排泄,则到达动态平衡;
若是产生>排泄,则尿酸程度升高,持久可能构成高尿酸血症;
若是产生<排泄,则尿酸程度过低,持久可能构成低尿酸血症。
尿酸排泄:肾收受接管90%大大都哺乳动物,都不间接排出尿酸。
它们体内有响应的基因,能够编码出一种卵白:尿酸酶(Uricase)。
尿酸酶,能够把尿酸进一步氧化为尿囊素,那是一种消融度很高的物量,能够随便随尿液排出体外[104]。
也就是说,对大大都哺乳动物而言,尿酸只是代谢的中间产品,最末排出的是尿囊素。
我们人类/灵长类曾经也是如许,但是后来变了。
我们祖先的尿酸酶基因发作突变[2,21,22,119],使我们不克不及再产生尿酸酶[22],无法把尿酸氧化为尿囊素。
有趣的是,现代人和高档灵长类动物还保有非功用性尿酸酶基因(也叫伪基因)[1]。
我们不单不产生尿酸酶了,还用肾脏把尿液中的尿酸重吸收90%摆布[23,24],只要10%摆布排出体外。
向尿酸致敬!固然如今人类站在食物链高峰,在其他动物面前,我们像神一样掌握了生杀大权,但我们的祖先其实比力弱势。
在十分遥远的年代(6百万年以前甚至更早),我们的祖先是灵长类,次要住在树上。生物学家认为那是一个相对平安的区域,那就意味着,我们的祖先在食物链的位置中等偏下,需要依靠树木遁藏猛兽。
地位不高,意味着我们的祖先其实大大都时候,很难从动物性食物中获取脂肪。固然它们能抓到各类小型动物(青蛙,蜘蛛,轮虫,蛇,蜥蜴等),但那些小型动物的脂肪含量,凡是都很低(贝爷经常抓住它们,然后说高卵白低脂肪)。
所以,在远古的期间,约一千多万年前,我们的祖先是靠果糖来贮存脂肪的[3,4,125]。果糖在肝脏代谢,先转为果糖-1-磷酸,再转为三磷酸甘油或长链脂肪酸[111,112,113]。
如许我们的祖先就能在吃不到大型动物的前提下,只靠吃果子就获得脂肪,如许风险小,平安高效,对保存十分重要。
其实,不但是我们的祖先,良多草食动物都靠果糖来贮存足够的脂肪过冬。
但后来情况变了,在1200-1400万年前发作了“中新世纪灾难”(Middle Miocene DisRU)[125],全球气温大幅度下降,大量动动物灭亡,我们祖先的食物大量削减。
怎么办呢?操纵尿酸,收受接管尿酸。
因为尿酸能够激活一个过程,把相对不容易转化为脂肪的葡萄糖,也转化为果糖[118]。
步调是:
(1)尿酸激活AR(醛糖复原酶)[116,117];
(2)AR 将葡萄糖转为山梨醇;
(3)山梨醇在山梨醇脱氢酶(SDH)的感化下,转化为果糖;
如许,葡萄糖就在尿酸的感化下,成了果糖[114,115];
接下来,我们的祖先再把果糖转为脂肪,美滋滋。
更棒的是,果糖代谢为脂肪的过程中,因为能量代谢的问题,又会产生更多的嘌呤。
(我们在文章开头说了,嘌呤既是核酸的构成部门,也是ATP代谢的产品)
更多的嘌呤,意味着更多的尿酸。
所以,那就成了一个轮回:
(1)果糖转化脂肪的过程中,产生嘌呤-尿酸;
(2)尿酸又把更多的葡萄糖转化为果糖;
(3)果糖再转化为脂肪,过程再产生尿酸......
如斯轮回往复,就到达了脂肪产量的更大化[125]。
那就是我们的祖先要“烧毁”尿酸酶的原因,因为尿酸有个重要感化:促进脂肪堆积[5,6]。
Laura等人证明了尿酸促进脂肪堆积那件事。
他们用尿酸来处置细胞(HepG2细胞),发现尿酸能进步一些酶的活性(SDH酶等等);那些酶的活性进步,能够促进葡萄糖向果糖的转化,从而进步甘油三酯在体内(出格是脂肪)的积累[9,,127,128]。
所以,我们的祖先身上发作的尿酸酶基因突变(失去了尿酸酶),不再把尿酸转化为尿囊素排出,而是收受接管尿酸,用来获取更多的脂肪贮存[125,127,128]。
演化,是为了保存。
更令人服气的是,尿酸的感化不只限于此,还有维持血压的感化。
固然维持血压,在我们现代人看来不是个事,但是在远古时候可不那么容易,因为那时候没有造盐手段,很难吃到足够的盐,就不容易维持血压(那就是为什么人体要保钠排钾)。
还有,当较长时间食物匮乏、饥饿时等,哺乳动物容易发作低血压[17,18,19],所以尿酸维持血压的感化就显得出格重要。
尿酸通过一氧化氮来影响内皮血管功用[10,11,12,13,14],或是影响肾素-血管严重素系统[15,16]来维持血压。
留意,果糖-尿酸-脂肪的相关代谢,都在肝脏发作,因而喝了太多的甜饮料(含有许多果糖)容易诱发脂肪肝;尿酸高的人别喝甜饮料和果糖,那是常识。
但是换个角度想,若是不是那套机造,我们的祖先可能早就因为缺乏脂肪和能量,冻死、饿死在中新世纪灾难的雪窖冰天里了。
那么,也就提早“全剧末”,哪还有现代的人类文明,哪还有拿动手机刷知乎的我们?
向尿酸致敬!
尿酸对神经/大脑有必然庇护感化因为我们重吸收了90%摆布的尿酸,所以招致人类/灵长类比其他哺乳动物血液中的尿酸程度高了接近10倍[46,47,120,121]。
人类的演化是沿着一条“智力属性”的道路走的,所以大脑和神经对我们来说出格重要。
Havelock Ellis等人很早就提出,尿酸可能与人类的智力程度有关[126]。有些研究者认为,人类比其他哺乳动物尿酸程度高,可能会是我们比它们更长命的原因之一[48]。
因为尿酸还有抗氧化和庇护神经的感化。
(留意,尿酸也能释放ROS活性氧,具有双重身份)
有利的研究显示,尿酸约占血浆抗氧化活性的60%,能维持血压和抗氧化应激的不变性[25,26];尿酸能肃清氧自在基[122],避免维生素C在体内被氧化[123,124];
心理浓度下的尿酸能降低人体内的一些过氧化反响,庇护红细胞[20]、避免低密度脂卵白的氧化和超氧化物歧化酶的失活[27]。
尿酸能够多形核血和红细胞产生的自在基所介导的器官损伤[142],能够庇护重要的抗氧化酶——超氧化歧化酶的构造和功用[143,144],能够庇护DNA削减氧化损伤[145];
对包罗了安康人、抽烟者、1型糖尿病患者和心血管疾病患者在内的差别人群的研究数据表白,对血液中输入尿酸,可显著改善总抗氧化才能和保留血管内皮功用[146,147,148,149,150].
有趣的是,不单一般浓度的尿酸对人体有庇护感化,高尿酸血症也对一些神经系统疾病如阿兹海默症有庇护感化[7,30,43];
反过来,低尿酸血症与神经系统疾病亲近相关[7],可增加老年痴呆症、帕金森病、多发性硬化症、精神团结症和痴呆症的风险[44,45,46]。
所以,尿酸酶基因的丧失、人体对尿酸的收受接管,是精打细算的成果。
高尿酸的关键:排泄不顺畅前瞻性流行病学研究指出,尿酸升高的病理机造复杂,与许多疾病/亚安康形态有关,如氧化应激、瘦削、高血压、代谢综合征、慢性肾病等[73,74,75,76,77,78];
此外,不良生活习惯,久坐、熬夜、铅、血液中铁含量过高档良多因素也可招致尿酸升高。
但是归根到底,尿酸程度是取决于尿酸合成(包罗食物摄入)和尿酸排泄的差值[72]。
那尿酸高,到底是尿酸生成太多,仍是尿酸排泄不顺畅?
我们能看到一些证据:
肾功用一般的人食用富含嘌呤的食物后,尿酸排泄增加50%以上[83];
高尿酸血症患者的尿酸肃清率和排泄量都比一般人低[85];
在瘦削、胰岛素抵御和高血压患者中,尿酸的升高次要是因为肾排泄受损所致[42,87];
目前支流科学认为,尿酸排泄不顺畅(而非摄入/合成过多)是高尿酸问题的主因[79,80,81,82,84]。
为什么排泄不顺畅?遗传因素按照我国的一项研究[86],高尿酸的遗传力为0.63(1暗示完全由遗传决定,0暗示完全由情况因素决定),那表白遗传因素是尿酸高的主因。
Adrienne等人对457690人停止了跨种族血清尿酸的阐发,确定了183个与尿酸代谢相关的基因点位[89],也申明遗传对尿酸程度具有深远影响。
遗传对尿酸程度的影响,次要是通过各类转运尿酸的卵白来实现的。
各人都晓得,肾脏重吸收了每天尿液中90%的尿酸,那种重吸收,非常依靠上述卵白;那些卵白也有本身的DNA,它们受遗传突变、基因表达差别所影响。
(打个例如,若是那些卵白是运载尿酸的货车,那么DNA就是造造那些货车的工场,货车的数量和性能取决于工场的产量和消费量量)
我们看几种典型的转运卵白:
第一种,ABCG2,全名是ATP binding cassette subfamily member2,它是一种尿酸转运体卵白[66,67,68],位于肾、肠等组织的根尖细胞膜上,它依赖ATP做为能量,运载各类化合物。
ABCG2对尿酸在肾脏和肠道中的排泄起重要感化[50,51,52]。它发作功用障碍,会招致肾脏对尿酸的排泄显著削减,那被认为是招致高尿酸血症的次要原因之一。
Ichida等人研究了了664名男性高尿酸患者[53],发如今那些人中,71.9%的人都发作了ABCG2基因的突变(突变的是ABCG2上的一个叫Q141K的点位),那申明高尿酸患者中遗传突变是比力遍及的。
Matsuo等人对90例高尿酸血症患者的ABCG2基因停止了突变阐发[52],发现6六个突变点位。
它们关于尿酸程度有显著影响:例如ABCG2基因上的Q126X点位突变,可招致痛风风险大幅度提拔,增加597%。
那6个点位,鄙人图中是橙色字体。
天经地义的是,若是ABCG2呈现功用障碍,也与慢性肾病存在亲近关系[54,55,56,56,57]。
此外,ABCG2不但在尿酸的肾脏排泄路子中有核心地位,在肠道尿酸排泄中也是一种重要的转运体[52]。
有趣的是,若是若是肾尿酸排泄削减,ABCG2的肠道表达就会增加。
也就是说,当肾脏不克不及排泄足够的尿酸,人体味试图增加肠道对尿酸的排泄,那在慢性肾病中出格重要,被称为“长途器官通信”[58]。
第二种,URAT1,中文名为尿酸转运体1[61],是基因SLC22A12编码的产品,它是含有553个氨基酸的转运卵白37,38,39,40]。
它与另一种名为“钠氢交换调理剂”的卵白连系感化,在肾脏对尿酸的重吸收中阐扬重要感化[37,47,48]。
URAT1的基因突变会招致URAT1功用失调,那被认为是高尿酸血症的次要原因[41,42]。
有趣的是,一项中国的研究发现,人类URAT1基因的启动子中,有雄激素反响元件[82]。
什么是激素反响元件?
我们都晓得,激素需要受体,但并非跟受体连系就完事了。
激素和受体连系后,还需要与DNA上的一段区域连系,才气影响DNA——因为卵白量是DNA表达(造造)出来的。
DNA上的那段区域,就是激素反响元件。
就尿酸来说,睾酮与受体连系后,坐落于URAT1基因上,加强其表达。
更强的URAT1表达意味着男性的URAT1的性能更强,对尿酸的收受接管更彻底。
那在很大水平上解释了为什么男性比女性的尿酸程度更高;所以原做者更间接的说,那是男女之间尿酸不同的底子原因。
第三种,GLUT9,中文名葡萄糖转运卵白9[62,63,64],是基因SLC2A9编码的产品,其基因普遍存在于人的肾小管近端[8],有a/b两个亚型[28]。
GLUT9最早被认为是转运葡萄糖用的,所以得了个“葡萄糖转运卵白”的名字。
但是后来发现,它在尿酸重吸收过程中阐扬了关键感化[31],因而GLUT9的遗传/功用缺陷可招致高尿酸血症[88]。
有趣的是,遗传缺陷不单可能招致高尿酸血症,也可能反过来也能够招致GLUT9过少,肾小管吸收尿酸太少,招致低尿酸血症[32,33,34]。
尿酸少了欠好吗?
是的,我们人类需要心理浓度的尿酸(血压,庇护神经系统,抗氧化,维持氧化应激稳态等)。
若是尿酸过少,除了容易引发神经系统病变[43,49],还可能引发肾结石和肾衰竭[35,36]——是的,你没听错,过低的尿酸也可能引起肾病。
参与尿酸代谢的转运体还有良多,我们就纷歧一介绍了。
上述常识解释了为什么同样是海鲜啤酒,有些人尿酸到达700,800多,有些人却照旧连结在400多:因为遗传因素影响了上述转运卵白的数量、活性、功用等,形成了肾脏对尿酸排泄才能的差别。
孕期/少小饥荒寡所周知,1959-1961年中国遭遇了三年天然灾祸和大饥荒。
张文强等人研究了中国安康与退休纵向研究”(CHARLS)的数据,那些数据中包罗了天然灾祸期间出生的2383人[127]。
那些数据指向,在怀孕期间/少小表露于饥荒中并在中老年发作瘦削的人,发作高尿酸血症的几率进步三倍以上。
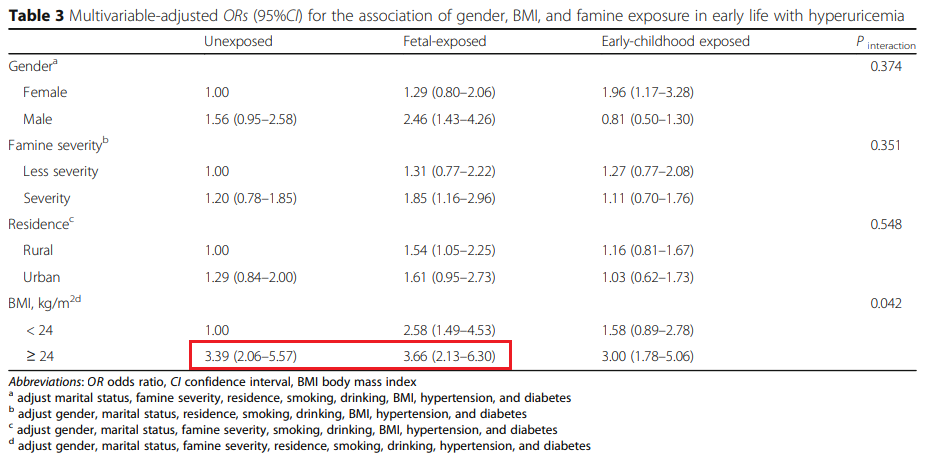
类似的结论也有其他许多研究撑持[134,135,136,137],胎儿期或早年发作营养不良,发作高尿酸血症和瘦削/胰岛素血症的几率更大。
其原因被是胎儿在子宫内营养不良、低出生体重低,可能招致肾单元数较少[128,129],那招致在中老年后肾脏功用下降较快[130,131,132,133];
早期营养不良的人也有更大的几率瘦削,瘦削招致的胰岛素抵御也会削减尿酸排泄,从而间接促成高尿酸血症[138,139,140,141]。
久坐在现代社会,一般人清醒的大部门时间都坐着,但久坐带来的问题是多方面的:
在心血管疾病患者中,久坐是形成所有类型灭亡的一个强力的独立因素[90];
大量的体育活动能够避免体重增加,体育活动是预防瘦削的一个关键因素[91];
每天坐超越>10小时的人,比坐<1小时的,灭亡率超出跨越34%,久坐时间是一个影响安康的独立因素,无论能否瘦削[92];
更蹩脚的是,按照比来的研究,若是超越每日均匀坐时间,按期运动所供给的积极安康成果就会消逝,并可能招致慢性病的时机增加[93];
体力活动削减、久坐,也是升高尿酸的因素。
一项我国的荟萃阐发(包罗11项研究,63689人)表白:低/中等强度体力活动会降低高尿酸风险:男性12%/女性1%;高强度体力活动降低高尿酸风险:男性29%/女性32%。
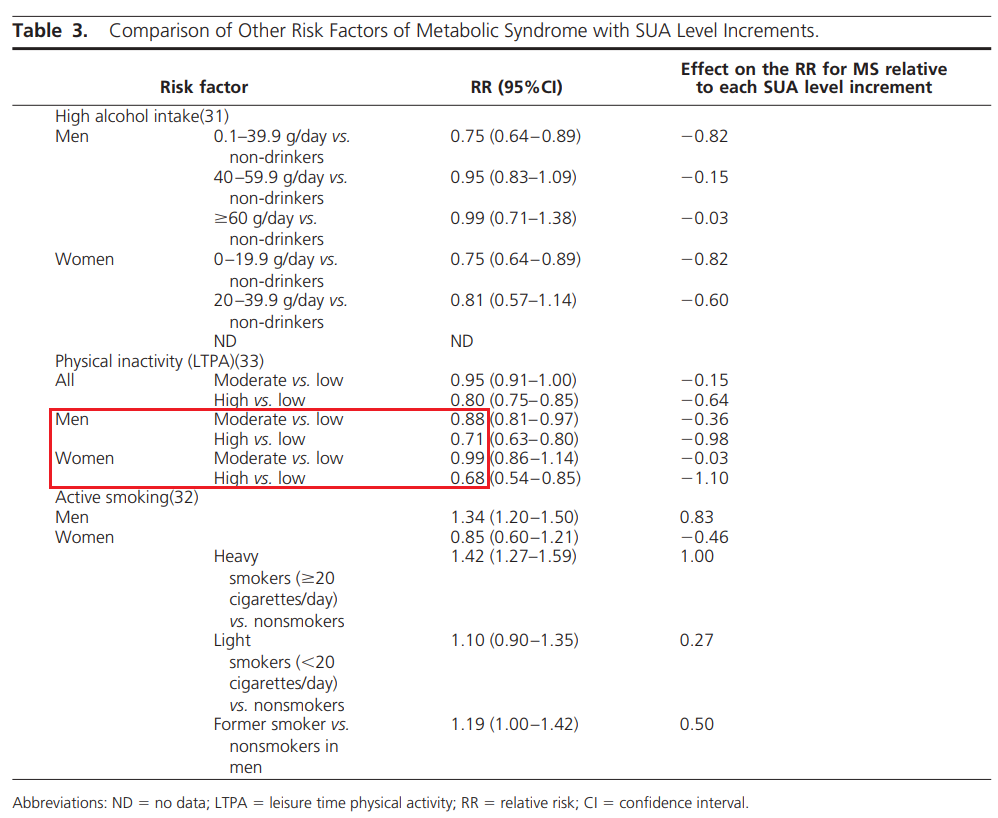
其可能的机造是:
胰岛素会促进钠在肾小管的吸收,从而削减尿酸的排放[95],因而胰岛素抵御/瘦削可能升高血尿酸程度[96,97];久坐会削减能量消耗[100,101],削减肌肉中的葡萄糖和脂肪利用,从而增加胰岛抵御[102,103];而肌肉收缩、体力活动能够削减胰岛素抵御[98],运动自己也能够削减尿酸的生成[96,99]。
留意,番茄良多人容易轻忽番茄,认为番茄是低嘌呤食物,能够多吃。
番茄不断以来就被各地的陈述容易形成痛风。在一些报导中,番茄被痛风患者提到容易触发痛风,需要制止[105];在新西兰的痛风人群中,番茄摄入与痛风爆发频次正相关[106];
LEE等人发现吃了番茄酱后尿酸程度升高[107];
tanya等人研究确诊痛风的2051名毛利人发现,番茄摄入量确实与尿酸程度正相关[110]。
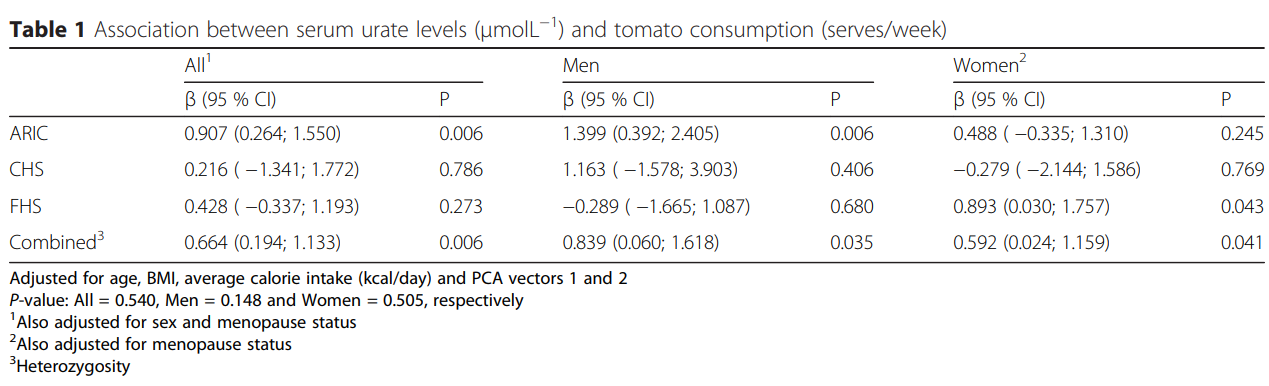
做者提出的解释是:
(1)固然番茄自己的嘌呤含量确实很低[108],但番茄中含有较高多的谷氨酸,能在人体嘌呤合成路子中充任氮的供给者,来刺激或放大尿酸的合成[108,109];
(2)番茄中含有一些化合物(p-coumaric acid、ferullic acid、p-hydroxybenzoic acid 、vanillic acid),那些化合物能够与OAT1、OAT3等肾脏中参与尿酸代谢的转运卵白彼此感化,从而影响肾脏对尿酸的排泄。
关于那一点,痛风或者高尿酸的伴侣能够出格留意,不然可能尿酸高了还不晓得为什么。
回到主题,为什么现代年轻人尿酸高?毫无疑问那是基因和情况配合感化的成果,此中基因起了相对更大的感化。
我们也能够理解为,现代年轻人经常饮食/做息/生活方面存在问题,或摄入嘌呤食物过多等等......那一切情况因素,感化于差别的遗传前提的人身上,成果是:
那些具有遗传易感性的人就会发作高尿酸;
而不具有遗传易感性的人,则不发作高尿酸、或者尿酸的上升较为轻细。
卵白粉和乳成品具有降尿酸、庇护肾脏的感化许多证据证明,高尿酸程度对肾脏病和代谢综合征有促进感化。
乳成品和卵白粉通过乳清酸等机造(它与尿酸合作阴离子),削减人体对尿酸的重吸收,增加尿酸排泄,从而起到降尿酸、庇护肾脏的感化。
肌酸跟卵白粉到底对肾脏有没有影响?
保举阅读:肉崽:力训研究所课程介绍
肉崽:力训研究所线上期刊介绍
健身5/6分化训练底子不合适通俗人肉崽:力训研究所新手指南(十二)为什么分化训练不合适通俗人(上)
肉崽:力训研究所新手指南(十三)为什么分化训练不合适通俗人(中)
肉崽:力训研究所新手指南(十四)为什么分化训练不合适通俗人(下)
新手卧推用手臂?老手才气用胸发力?卧推要感触感染胸肌才有效?假的肉崽:卧推不需要感触感染胸肌或专注于胸肌收缩
怎么找到哑铃荡舟背阔肌发力感?
练胸肌的时候,手臂力量经常跟不上怎么办?
训练后不拉伸,没有任何问题肉崽:训练后需要拉伸吗?(一)
肉崽:训练后需要拉伸吗?(二)
肉崽:训练后需要拉伸吗?(三)
肉崽:训练后需要拉伸吗:参考文献(1-200)
肉崽:训练后需要拉伸吗:参考文献(200-323)
健美训练中被人曲解的几大体素肉崽:被曲解最多的健美训练要素(一):轻重量切确刺激肌肉
肉崽:被曲解最多的健美训练要素(二):慢速动做
肉崽:被曲解最多的健美训练要素(三):孤立训练
肉崽:被曲解最多的健美训练要素(四):变更动做赐与肌肉新刺激
肉崽:被曲解最多的健美训练要素(五):根据肌纤维类型针对训练
肉崽:“8-12次更佳增肌次数范畴” 其实是个伪命题
动做细节=手艺?细节差别天崩地裂翻天覆地?纯属瞎掰。肉崽:动做细节的影响有多大?
飞鸟和夹胸练中缝,纯属虚构肉崽:胸肌中缝和铠甲胸是飞鸟练出来的吗?
练胸不练背,既不会改动身形,也不会“圆肩”肉崽:练胸不练背会圆肩?有几人受骗被骗?
并非训练让我们变强,而是训练激发了、发掘了你自己就有的潜力。那些健身模特是不是都在用类固醇?通俗人能不克不及练成如许?
训练增肌的核心原理,并非“练得微损伤后修复增粗”肉崽:力训研究所新手指南(八)增肌的深层原理
激素的增肌原理,其实跟训练是一样的既然只要卵白量才气转化肌肉为什么增肌期间还要摄入大量碳水?
生长激素到底可以增加肌纤维数量吗?
营养具有跟训练、激素类似的增肌感化为什么减脂容易掉肌肉?
不健身间接吃卵白粉会怎么样?
健身练到那种水平能够起头喝卵白粉?
人人都在说卵白量过量,但其实很难实现卵白量摄入过多的危害有哪些?
健身必然要喝卵白粉吗,还有健身危害好比说对肝脏?
肉崽:聊聊补剂商人本身都不晓得的左旋肉碱用途
高训练量是个坑,并且是大坑肉崽:堆训练量?3倍勤奋,事倍功半
肉崽:从一个高容量方案换成另一个高容量方案,为什么前进了?
肉崽:10X10改进版数据带给我们的启迪
同样的训练,有些人比他人增肌幅度大好几倍,不是因为手艺差别肉崽:训练潜力与遗传(一):卵白量与基因
肉崽:训练潜力与遗传(二):DNA的表达
肉崽:训练潜力与遗传(三):肌肉程度与细胞核
肉崽:训练潜力与遗传(四):肌肉程度与微RNA
肉崽:训练潜力与遗传(五):肌肉增长与睾酮受体
关于统一个训练系统来说,肌肉和力量成反比肉崽:力量举的“先天”次要指什么?
睡眠从分子角度若何影响肌肉的代谢“肌肉在歇息的时候超量生长”,此中关于“歇息”的定义是睡觉吗?
若是你每天睡眠时间只要6个小时,你能否愿意拿出半个小时熬炼身体?
为何一天1小时的力量+高强度无氧+1小时有氧跑,已对峙近半个月既没减脂又没增肌?
肉崽:健身与睡眠(三)
生酮饮食的效果与高碳饮食的减肥效果差别很小肉崽:生酮饮食(一):效果
肉崽:低碳生酮饮食(五)副感化:概略和灭亡风险
肉崽:低碳生酮饮食(七)副感化:肝损伤
肉崽:低碳生酮饮食(八)副感化:骨安康
肉崽:低碳生酮饮食(九)副感化:骨安康
肉崽:低碳生酮饮食(十一)肾结石和胆结石
肉崽:生酮饮食是若何损害心脏的?(上)
肉崽:生酮饮食是若何损害心脏的?(下)
小我理论没有任何说服力,“干就完了”“练得大说得对”都属于缺乏根本的逻辑和常识肉崽:健身喜好者更大的误区之一:“理论出实知”
肉崽:力咖新手指南(六)新手必需避开的认知雷区:(上)
肉崽:力咖新手指南(六)新手必需避开的认知雷区:(中)
营养/恢复相关不健身间接吃卵白粉会怎么样?
健身一年没有什么前进,一般吗?
为什么减脂容易掉肌肉?
既然只要卵白量才气转化肌肉为什么增肌期间还要摄入大量碳水?
为何一天1小时的力量+高强度无氧+1小时有氧跑,已对峙近半个月既没减脂又没增肌?
为什么都说增肌要碳水?
现代年轻报酬什么尿酸会高?
肌酸跟卵白粉到底对肾脏有没有影响?
References1. Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19(5):640–653.
2. Masako Oda, Yoko Satta, Osamu Takenaka, Naoyuki Takahata.Loss of urate oxidase activity in hominoids and its evolutionary implications.Mol Biol Evol. 2002 May;19(5):640-53.
3. Ackerman Z., Oron-Herman M., Grozovski M., Rosenthal T., Pappo O., Link G., and Sela B. A. (2005) Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 45, 1012–1018.
4. Ishimoto T., Lanaspa M. A., Le M. T., Garcia G. E., Diggle C. P., Maclean P. S., Jackman M. R., Asipu A., Roncal-Jimenez C. A., Kosugi T., Rivard C. J., Maruyama S., Rodriguez-Iturbe B., Sánchez-Lozada L. G., Bonthron D. T., et al. (2012) Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. U.S.A. 109, 4320–4325
5. Lanaspa M. A., Sanchez-Lozada L. G., Choi Y. J., Cicerchi C., Kanbay M., Roncal-Jimenez C. A., Ishimoto T., Li N., Marek G., Duranay M., Schreiner G., Rodriguez-Iturbe B., Nakagawa T., Kang D. H., Sautin Y. Y., et al. (2012) Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287, 40732–40744
6. Lanaspa M. A., Cicerchi C., Garcia G., Li N., Roncal-Jimenez C. A., Rivard C. J., Hunter B., Andrés-Hernando A., Ishimoto T., Sánchez-Lozada L. G., Thomas J., Hodges R. S., Mant C. T., and Johnson R. J. (2012) Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One 7.
7. Tana C., Ticinesi A., Prati B., Nouvenne A., Meschi T. (2018). Uric Acid and Cognitive Function in Older Individuals. Nutrients 10, 97
8. Kimura T., Takahashi M., Yan K., Sakurai H. (2014). Expression of SLC2A9 Isoforms in the Kidney and Their Localization in Polarized Epithelial Cells. PLoS One 9, e84996.
9. Laura G. Sanchez-Lozada1 , Ana Andres-Hernando2 , Fernando E. Garcia-Arroyo1 , Christina Cicerchi2 , Nanxing Li2 , Masanari Kuwabara2 , Carlos A. Roncal-Jimenez2 , Richard J. Johnson2 and Miguel A. Lanaspa 2* . Uric acid
activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats.JBC Papers in Press. Published on January 16, 2019 as Manuscript RA118.006158.
10. Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183–1190.
11. Mene P, Punzo G. Uric acid: bystander or culprit in hypertension and progressive renal disease? Journal of Hypertension. 2008;26(11):2085–2092.
12. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney International. 2005;67(5):1739–1742.
13. Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106(2):221–226.
14. Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619–2624.
15. Toma I, Kan J, Meer E, Pet-Peterdi J. Uric acid triggers renin release via a macula densa-dependent pathway. Presented at: American Society of Nephrology Annual Meeting; San Francisco, CA. 2007. F-P0240.
16. Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, et al. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney International. 2004;66(4):1465–1470.
17. S. E. Kocyigit, P. Soysal, E. Ates Bulut & A. T. Isik.Malnutrition and Malnutrition Risk Can Be Associated with Systolic Orthostatic Hypotension in Older Adults.The journal of nutrition, health & aging volume 22, pages 928–933 (2018).
18. Joel B Gunter.Fasting, halothane, and hypotension.Anesth Analg. 2003 May;96(5):1537-8; author reply 1538.
19. T D Williams 1 , J B Chambers, R P Henderson, M E Rashotte, J M Overton.Cardiovascular responses to caloric restriction and thermoneutrality in C57BL/6J mice.Am J Physiol Regul Integr Comp Physiol. 2002 May;282(5):R1459-67.
20. B N Ames, R Cathcart, E Schwiers, and P Hochstein.Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis.Proc Natl Acad Sci U S A. 1981 Nov; 78(11): 6858–6862.
21. Masako Oda, Yoko Satta, Osamu Takenaka, Naoyuki Takahata.Loss of urate oxidase activity in hominoids and its evolutionary implications.Mol Biol Evol. 2002 May;19(5):640-53.
22. Fatemeh Dabbagh, Mohammad B Ghoshoon, Shiva Hemmati, Mozhdeh Zamani, Milad Mohkam, Younes Ghasemi.Engineering Human Urate Oxidase: Towards Reactivating It as an Important Therapeutic Enzyme.Curr Pharm Biotechnol.2015;17(2):141-6.
23. Jessica Maiuolo, Francesca Oppedisano, Santo Gratteri, Carolina Muscoli, Vincenzo Mollace.Regulation of uric acid metabolism and excretion.Int J Cardiol. 2016 Jun 15;213:8-14.
24. Jessica Maiuolo, Gratteri S, Muscoli C, Mollace V.(2016). Regulation of Uric Acid Metabolism and Excretion. Int.J.Cardiol.15,8–14.
25. Nieto C. I. F. J., Gross M. D., Comstock G. W., Cutler R. G. (2000). Uric Acid and Serum Antioxidant Capacity: a Reaction to Atherosclerosis? Atherosclerosis 148, 131–139.
26. Wang Q., Wen X., Kong J. (2020). Recent Progress on Uric Acid Detection: A Review. Crit. Rev. Anal Chem. 50, 359–375.
27. Bassanese G., Wlodkowski T., Servais A., Heidet L., Roccatello D., Emma F., et al. (2021). The European Rare Kidney Disease Registry (ERKReg): Objectives, Design and Initial Results. Orphanet J. Rare Dis. 16, 251.
28. So A., Thorens B. (2010). Uric Acid Transport and Disease. J. Clin. Invest 120, 1791–1799.
29. Bardin T., Richette P. (2014). Definition of Hyperuricemia and Gouty Conditions. Curr. Opin. Rheumatol. 26, 186–191.
30. Latourte, Augustina,b; Bardin, Thomasa,b; Richette, Pascala,b.Uric acid and cognitive decline: a double-edge sword?.Current Opinion in Rheumatology: March 2018 - Volume 30 - Issue 2 - p 183-187.
31. Ebert K., Ludwig M., Geillinger K. E., Schoberth G. C., Essenwanger J., Stolz J., et al. (2017). Reassessment of GLUT7 and GLUT9 as Putative Fructose and Glucose Transporters. J. Membr. Biol. 250, 171–182.
32. Akaoka I, Nishizawa T, Yano E, Takeuchi A, Nishida Y. Familial hypouricaemia due to renal tubular defect of urate transport. Ann Clin Res. 1975;7(5):318–324.
33. Wakasugi M, Kazama JJ, Narita I, Konta T, Fujimoto S, Iseki K, et al. Association between hypouricemia and reduced kidney function: a cross-sectional population-based study in Japan. Am J Nephrol. 2015;41(2):138–146.
34. Suzuki T, Kidoguchi K, Hayashi A. Genetic heterogeneity of familial hypouricemia due to isolated renal tubular defect. Jinrui Idengaku Zasshi. 1981;26(3):243–248.
35. Kikuchi Y., Koga H., Yasutomo Y., Kawabata Y., Shimizu E., Naruse M., Kiyama S., Nonoguchi H., Tomita K., Sasatomi Y. Patients with renal hypouricemia with exercise-induced acute renal failure and chronic renal dysfunction. Clin. Nephrol. 2000;53:467–472.
36. Diamond H.S., Paolino J.S. Evidence for a postsecretory reabsorptive site for uric acid in man. J. Clin. Invest. 1973;52:1491–1499.
37. Hosoyamada M., Ichida K., Enomoto A., Hosoya T., Endou H. (2004). Function and Localization of Urate Transporter 1 in Mouse Kidney. J. Am. Soc. Nephrol. 15, 261–268.
38. Enomoto A., Kimura H., Chairoungdua A., Shigeta Y., Jutabha P., Cha S. H., et al. (2002). Molecular Identification of a Renal Urate Anion Exchanger that Regulates Blood Urate Levels. Nature 417, 447–452.
39. Zhou F., Zhu L., Cui P. H., Church W. B., Murray M. (2010). Functional Characterization of Nonsynonymous Single Nucleotide Polymorphisms in the Human Organic Anion Transporter 4 (hOAT4). Br. J Pharmacol 159, 419–427.
40. Skwara P., Sch?mig E., Gründemann D. (2017). A Novel Mode of Operation of SLC22A11: Membrane Insertion of Estrone Sulfate versus Translocation of Uric Acid and Glutamate. Biochem. Pharmacol. 15, 74–82.
41. Zhu C., Sun Bao., Zhang B., Zhou Z. (2021). An Update of Genetics, Co-morbidities and Management of Hyperuricemia. Clin. Exp. Pharmacol. Physiol.
42. Pasquale Strazzullo, Juan Garcia Puig.Uric acid and oxidative stress: relative impact on cardiovascular risk?.Nutr Metab Cardiovasc Dis. 2007 Jul;17(6):409-14.
43. Mandal A. K., Mount D. B. (2019). Interaction between ITM2B and GLUT9 Links Urate Transport to Neurodegenerative Disorders.
44. Ascherio A., LeWitt P. A., Xu K., Eberly S., Watts A., Matson W. R., et al. (2009). Urate as a Predictor of the Rate of Clinical Decline in Parkinson Disease. Randomized Controlled Trial 66, 1460–1468.
45. Du N., Xu D., Hou X., Song X., Liu C., Chen Y., et al. (2016). Inverse Association between Serum Uric Acid Levels and Alzheimers Disease Risk. Mol. Neurobiol. 53, 2594–2599.
46. Ye B. S., Lee W. W., Ham J. H., Lee J. J., Lee P. H., Sohn Y. H. (2016). Does Serum Uric Acid Act as a Modulator of Cerebrospinal Fluid Alzheimers Disease Biomarker Related Cognitive Decline? Eur. J. Neurol. 23, 948–957.
47. Cunningham R., Brazie M., Kanumuru S., Xiaofei E., Biswas R., Wang F., et al. (2007). Sodium-hydrogen Exchanger Regulatory Factor-1 Interacts with Mouse Urate Transporter 1 to Regulate Renal Proximal Tubule Uric Acid Transport. J. Am. Soc. Nephrol. 18, 1419–1425.
48. Shenolikar S., Voltz J. W., Minkoff C. M., Wade J. B., Weinman E. J. (2002). Targeted Disruption of the Mouse NHERF-1 Gene Promotes Internalization of Proximal Tubule Sodium-Phosphate Cotransporter Type IIa and Renal Phosphate Wasting. Proc. Natl. Acad. Sci. U S A. 99, 11470–11475.
49. Houlihan L. M., Wyatt N. D., Harris S. E., Hayward C., Gow A. J., Marioni R. E., et al. (2010). Variation in the Uric Acid Transporter Gene (SLC2A9) and Memory Performance. Hum. Mol. Genet. 19, 2321–2330.
50. Maliepaard M., Scheffer G. L., Faneyte I. F., van Gastelen M. A., Pijnenborg A. C., Schinkel A. H., et al. (2001). Subcellular Localization and Distribution of the Breast Cancer Resistance Protein Transporter in Normal Human Tissues. Cancer Res. 61, 3458–3464.
51. Yun-Hong Lu Y.-P. C., Li T., Han F., Li C-J., Li X-Y., Xue M., et al. (2020). Empagliflozin Attenuates Hyperuricemia by Upregulation of ABCG2 via AMPK/AKT/CREB Signaling Pathway in Type 2 Diabetic Mice. Int. J. Biol. Sci. 16, 529–542.
52. Matsuo H., Takada T., Ichida K., Nakamura T., Nakayama A., Ikebuchi Y., et al. (2009). Common Defects of ABCG2, a High-Capacity Urate Exporter, Cause Gout: A Function-Based Genetic Analysis in a Japanese Population. Sci. Transl Med. 1, 5ra11.
53. Ichida K., Matsuo H., Takada T., Nakayama A., Murakami K., Shimizu T., et al. (2012). Decreased Extra-renal Urate Excretion Is a Common Cause of Hyperuricemia. Nat. Commun. 3, 764.
54. Yano H., Tamura Y., Kobayashi K., Tanemoto M., Uchida S. (2014). Uric Acid Transporter ABCG2 Is Increased in the Intestine of the 5/6 Nephrectomy Rat Model of Chronic Kidney Disease. Clin. Exp. Nephrol. 18, 50–55.
55. Nigam S. K. (2015). What Do Drug Transporters Really Do? Nat. Rev. Drug Discov. 14, 29–44.
56. Bhatnagar V., Richard E. L., Wu W., Nievergelt C. M., Lipkowitz M. S., Jeff J., et al. (2016). Analysis of ABCG2 and Other Urate Transporters in Uric Acid Homeostasis in Chronic Kidney Disease: Potential Role of Remote Sensing and Signaling. Clin. Kideny J. 9, 444–453.
57. Cleophas M. C., Joosten L. A., Stamp L. K., Dalbeth N., Woodward O. M., Merriman T. R. (2017). ABCG2 Polymorphisms in Gout: Insights into Disease Susceptibility and Treatment Approaches. Pharmgenomics Pers Med. 10, 129–142.
58. Nigam S. K., Bhatnagar V. (2018). The Systems Biology of Uric Acid Transporters: the Role of Remote Sensing and Signaling. Curr. Opin. Nephrol. Hypertens. 27, 305–313.
59. Sorensen L. B. Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum. 8, 694–706 (1965).
60. Sica D. A. & Schoolwerth A. in Brenner and Rectors The Kidney (ed. B.M. Brenner) 645–649 (Saunders, 2004).
61. Enomoto A. et al.. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417, 447–452 (2002).
62. Li S. et al.. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 3, e194 (2007).
63. D?ring A. et al.. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 40, 430–436 (2008).
64. Vitart V. et al.. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 40, 437–442 (2008).
65. Anzai N. et al.. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 283, 26834–26838 (2008).
66. Dehghan A. et al.. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 372, 1953–1961 (2008).
67. Kolz M. et al.. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 5, e1000504 (2009).
68. Kamatani Y. et al.. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat. Genet. 42, 210–215 (2010).
69. X. W. Wu, C. C. Lee, D. M. Muzny, C. T. Caskey, Urate oxidase: Primary structure and evolutionary implications. Proc. Natl. Acad. Sci. U.S.A. 86, 9412–9416 (1989).
70. C. C. Lee, X. W. Wu, R. A. Gibbs, R. G. Cook, D. M. Muzny, C. T. Caskey, Generation of cDNA probes directed by amino acid sequence: Cloning of urate oxidase. Science 239, 1288–1291 (1988).
71. R. G. Cutler, Urate and ascorbate: Their possible roles as antioxidants in determining longevity of mammalian species. Arch. Gerontol. Geriatr. 3, 321–348 (1984).
72. Robinson PC. Gout-an update of aetiology, genetics, co-morbidities and management. Maturitas. 2018;118:67–73.
73. Wang H, Zhang H, Sun L, Guo W. Roles of hyperuricemia in metabolic syndrome and cardiac-kidney-vascular system diseases. Am J Transl Res. 2018;10:2749–2763.
74. Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40:155–175.
75. Ni Q, Lu X, Chen C, Du H, Zhang R. Risk factors for the development of hyperuricemia: a STROBE-compliant cross-sectional and longitudinal study. Medicine (Baltimore) 2019;98:e17597.
76. Chaudhary NS, Bridges SL Jr, Saag KG, Rahn EJ, Curtis JR, Gaffo A, Limdi NA, Levitan EB, Singh JA, Colantonio LD, Howard G, Cushman M, Flaherty ML, Judd S, Irvin MR, Reynolds RJ. Severity of hypertension mediates the association of hyperuricemia with stroke in the REGARDS case cohort study. Hypertension. 2020;75:246–256.
77. Steiger S, Ma Q, Anders HJ. The case for evidence-based medicine for the association between hyperuricaemia and CKD. Nat Rev Nephrol. 2020;16:422.
78. Sato Y, Feig DI, Stack AG, Kang DH, Lanaspa MA, Ejaz AA, Sanchez-Lozada LG, Kuwabara M, Borghi C, Johnson RJ. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol. 2019;15:767–775.
79. Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003;349:1647–1655.
80. Becker MA, Jolly M. In: Arthritis and Allied Conditions. 15. Koopman WJ, Moreland LW, editor. Philadelphia: Lippincott, Williams & Wilkins; 2005. Clinical gout and the pathogenesis of hyperuricemia; pp. 2303–2339.
81. Wyngaarden JB, Kelley WN. Gout and Hyperuricemia. New York: Grune & Stratton; 1976. pp. 1–512.
82. Li T, Walsh JR, Ghishan FK, Bai L. Molecular cloning and characterization of a human urate transporter (hURAT1) gene promoter. Biochim Biophys Acta 2004; 1681:53–58.
83. Fellstrom B., Danielson B.G., Karlstrom B., Lithell H., Ljunghall S., Vessby B. The influence of a high dietary intake of purine-rich animal protein on urinary urate excretion and supersaturation in renal stone disease. Clin. Sci. 1983;64:399–405.
84. Dalbeth N., Merriman T.R., Stamp L.K. Gout. Lancet. 2016;388:2039–2052.
85. Perez-Ruiz F., Calabozo M., Erauskin G.G., Ruibal A., Herrero-Beites A.M. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum. 2002;47:610–613.
86. Qiong Yang, Chao-Yu Guo, L Adrienne Cupples, Daniel Levy, Peter W F Wilson, Caroline S Fox.Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study.Metabolism. 2005 Nov;54(11):1435-41.
87. Juan García Puig 1 , María Angeles Martínez.Hyperuricemia, gout and the metabolic syndrome.Curr Opin Rheumatol. 2008 Mar;20(2):187-91.
88. Veronique Vitart et al.SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout.Nat Genet. 2008 Apr;40(4):437-42.
89. Adrienne Tin.et al.Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels.Nat Genet. 2019 Oct; 51(10): 1459–1474.
90. US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. DIANE Publishing; 1996.
91. Katzmarzyk P, Janssen I, Ardern C. Physical inactivity, excess adiposity and premature mortality. Obesity Rev. 2003;4(4):257–290.
92. Chau JY, Grunseit AC, Chey T, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One. 2013;8(11):e80000.
93. Thyfault JP, Du M, Kraus WE, et al. Physiology of sedentary behavior and its relationship to health outcomes. Med Sci Sports Exerc. 2015;47(6):1301.
94. Yuan H, Yu C, Li X, et al. Serum uric acid levels and risk of metabolic syndrome: a dose-response meta-analysis of prospective studies. J Clin Endocrinol Metab. 2015;100(11):4198–4207.
95. Facchini F, Chen Y-DI, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. Jama. 1991;266(21):3008–3011.
96. Katzmarzyk P, Janssen I, Ardern C. Physical inactivity, excess adiposity and premature mortality. Obesity Rev. 2003;4(4):257–290.
97. Chau JY, Grunseit AC, Chey T, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One. 2013;8(11):e80000.
98. Bouchard C, Blair SN, Katzmarzyk PT, et al. Less sitting, more physical activity, or higher fitness? Mayo Clinic Proc. 2015. Elsevier.
99. Wannamethee SG, Shaper AG, Alberti KGM. Physical activity, metabolic factors, and the incidence of coronary heart disease and type 2 diabetes. Arch Intern Med. 2000;160(14):2108–2116.
100. Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low‐intensity activity. J Physiol. 2003;551(2):673–682.
101. Hamilton TM, Hamilton GD, Zderic WT. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. 2004;32(4):161–166.
102. Hu FB, Leitzmann MF, Stampfer MJ, et al. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. (Original Investigation). Arch Internal Med. 2001;161(12):1542.
103. Hu F, Li T, Colditz G, Willett W, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791.
104. Lee I.R., Yang L., Sebetso G., Allen R., Doan T.H., Blundell R., Lui E.Y., Morrow C.A., Fraser J.A. Characterization of the complete uric acid degradation pathway in the fungal pathogen Cryptococcus neoformans. PLoS ONE. 2013;8:e64292.
105. Shulten P, Thomas J, Miller M, Smith M, Ahern M. The role of diet in the management of gout: a comparison of knowledge and attitudes to current evidence. J Hum Nutr Diet. 2009;22:3–11.
106. Winnard D, Wright C, Taylor WJ, Jackson G, Te Karu L, Arroll B, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology (Oxford) 2012;51:901–909.
107. Lee C-YJ, Isaac HB, Huang SH, Long LH, Wang H, Gruber J, et al. Limited antioxidant effect after consumption of a single dose of tomato sauce by young males, despite a rise in plasma lycopene. Free Radic Res. 2009;43:622–628.
108. Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Lanaspa MA, Tamura Y, Tanabe K, et al. Umami: the taste that drives purine intake. J Rheumatol. 2013;40:1794–1796.
109. Raivio KO, Seegmiller JE. Role of glutamine in purine synthesis and in guanine nucleotide formation in normal fibroblasts and in fibroblasts deficient in hypoxanthine phosphoribosyltransferase activity. Biochim Biophysic Acta. 1973;299:283–292.
110. Tanya J Flynn, Murray Cadzow, Nicola Dalbeth, Peter B Jones, Lisa K Stamp, Jennie Harré Hindmarsh, Alwyn S Todd, Robert J Walker, Ruth Topless, and Tony R Merrimancorresponding author.Positive association of tomato consumption with serum urate: support for tomato consumption as an anecdotal trigger of gout flares.BMC Musculoskelet Disord. 2015; 16: 196.
111. Maersk M., Belza A., Stødkilde-Jorgensen H., Ringgaard S., Chabanova E., Thomsen H., Pedersen S. B., Astrup A., and Richelsen B. (2012) Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am. J. Clin. Nutr. 95, 283–289
112. Ackerman Z., Oron-Herman M., Grozovski M., Rosenthal T., Pappo O., Link G., and Sela B. A. (2005) Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 45, 1012–1018 10.1161/01.HYP.
113. Ishimoto T., Lanaspa M. A., Le M. T., Garcia G. E., Diggle C. P., Maclean P. S., Jackman M. R., Asipu A., Roncal-Jimenez C. A., Kosugi T., Rivard C. J., Maruyama S., Rodriguez-Iturbe B., Sánchez-Lozada L. G., Bonthron D. T., et al. (2012) Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. U.S.A. 109, 4320–4325.
114. Lanaspa M. A., Ishimoto T., Li N., Cicerchi C., Orlicky D. J., Ruzycki P., Rivard C., Inaba S., Roncal-Jimenez C. A., Bales E. S., Diggle C. P., Asipu A., Petrash J. M., Kosugi T., Maruyama S., et al. (2013) Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 4, 2434
115. Lanaspa M. A., Kuwabara M., Andres-Hernando A., Li N., Cicerchi C., Jensen T., Orlicky D. J., Roncal-Jimenez C. A., Ishimoto T., Nakagawa T., Rodriguez-Iturbe B., MacLean P. S., and Johnson R. J. (2018) High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. U.S.A. 115, 3138–3143
116. Na K. Y., Woo S. K., Lee S. D., and Kwon H. M. (2003) Silencing of TonEBP/NFAT5 transcriptional activator by RNA interference. J. Am. Soc. Nephrol. 14, 283–288.
117. Woo S. K., Lee S. D., and Kwon H. M. (2002) TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 444, 579–585
118. Lanaspa M. A., Sanchez-Lozada L. G., Cicerchi C., Li N., Roncal-Jimenez C. A., Ishimoto T., Le M., Garcia G. E., Thomas J. B., Rivard C. J., Andres-Hernando A., Hunter B., Schreiner G., Rodriguez-Iturbe B., Sautin Y. Y. et al. (2012) Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One 7, e47948
119. Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution.J Mol Evol.1992;
120. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging.Proc Natl Acad Sci U S A.1993;90:7915–22.
121. Becker BF. Towards the physiological function of uric acid.Free Radic Biol Med.1993;14:615–31.
122. Maples KR, Mason RP. Free radical metabolite of uric acid.J Biol Chem.1988;263:1709–12.
123. Sevanian A, Davies KJ, Hochstein P. Conservation of vitamin C by uric acid in blood.J Free Radic Biol Med.1985;1:117–24.
124. Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid.Biochem J.1986;235:747–54.
125. Johnson RJ, Andrews P. Fructose, Uricase, and the Back to Africa Hypothesis. Evol Anthro. 2010 in press.
126. Ellis H. A study of British genius. London: Hurst and Blackett; 1903
127. Wenqiang Zhang and Rongsheng Luan.Early-life exposure to the Chinese famine of 1959–61 and risk of Hyperuricemia: results from the China health and retirement longitudinal study BMC Public Health (2020) 20:15.
128. Mesquita FF, Gontijo JA, Boer PA. Maternal undernutrition and the offspring kidney: from fetal to adult life. Braz J Med Biol Res. 2010;43(11):1010–1018.
129. Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr
CompPhysiol. 2003;285(5):R962–R970.
130. Franco M. Intrauterine undernutrition—renal and vascular origin of hypertension. Cardiovasc Res. 2003;60(2):228–234.
131. Lennox WG. Increase of uric acid in the blood during prolonged starvation. J Am Med Assoc. 1924;82:602–604.
132. Murphy R, Shipman KH. Hyperuricemia during Total fasting: renal factors. Arch Intern Med. 1963;112:954–959.
133. Lennox WG. A study of the retention of uric acid during starvation. J Biol Chem. 1925;66:521–572.
134. Alderman MH, Davis RP. Hyperuricemia in starvation. P Soc Exp Biol Med. 1965;118(3):790–792.
135. Drenick EJ. Hyperuricemia, acute gout, renal insufficiency and urate nephrolithiasis due to starvation. Arthritis Rheum. 1965;8:988–997.
136. Lennox WG. Increase of uric acid in the blood during prolonged starvation. J Am Med Assoc. 1924;82:602–604.
137. Lloydmostyn RH, Lord PS, Glover R, West C, Gilliland IC. Uric acid metabolism in starvation. Ann Rheum Dis. 1970;29(5):553–555.
138. Quinones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E. Effect of insulin on uric acid excretion in humans. Am J Phys. 1995;268(1 Pt 1):E1–E5.
139. Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 1997;92(1):51–58.
140. Lim SM, Choi DP, Rhee Y, Kim HC. Association between obesity indices and insulin resistance among healthy Korean adolescents: the JS high school study. PLoS One. 2015;10(5):e0125238.
141. Yamashita S, Matsuzawa Y, Tokunaga K, Fujioka S, Tarui S. Studies on the impaired metabolism of uric acid in obese subjects: marked reduction of renal urate excretion and its improvement by a low-calorie diet. Int J Obes. 1986;10(4):255–264.
142. Becker B.F. Towards the physiological function of uric acid. Free Radic. Biol. Med. 1993;14:615–631.
143. Hink H.U., Fukai T. Extracellular superoxide dismutase, uric acid, and atherosclerosis. Cold Spring Harb. Symp. Quant. Biol. 2002;67:483–490.
144. Hink H.U., Santanam N., Dikalov S., McCann L., Nguyen A.D., Parthasarathy S., Harrison D.G., Fukai T. Peroxidase properties of extracellular superoxide dismutase: Role of uric acid in modulating in vivo activity. Arterioscler. Thromb. Vasc. Biol. 2002;22:1402–1408.
145. Simic M.G., Jovanovic S.V. Antioxidation mechanisms of uric acid. J. Am. Chem. Soc. 1989;111:5778–5782.
146. Waring W.S. Uric acid: An important antioxidant in acute ischaemic stroke. QJM. 2002;95:691–693.
147. Waring W.S., Convery A., Mishra V., Shenkin A., Webb D.J., Maxwell S.R. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin. Sci. (Lond.) 2003;105:425–430.
148. Waring W.S., Maxwell S.R., Webb D.J. Uric acid concentrations and the mechanisms of cardiovascular disease. Eur. Heart J. 2002;23:1888–1889.
149. Waring W.S., McKnight J.A., Webb D.J., Maxwell S.R. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–3132.
150. Waring W.S., Webb D.J., Maxwell S.R. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J. Cardiovasc. Pharmacol. 2001;38:365–371.
版权声明
本文仅代表作者观点,不代表木答案立场。
